Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias vol.42 no.2 Lisboa jun. 2019
https://doi.org/10.19084/rca.17471
ARTIGO
The in vitro control of Fusarium proliferatum by propolis ethanolic extracts
Extratos etanolicos de própolis no controle in vitro de Fusarium proliferatum
Fernanda S. Gregolin1, Solange M. Bonaldo1, Adilson P. Sinhorin2, Jéfferson L. Banderó3 and Carmen Wobeto2,*
1 Universidade Federal de Mato Grosso, Instituto de Ciências Agrárias e Ambientais, Sinop-MT, Brasil, CEP: 78550-728.
2 Universidade Federal de Mato Grosso, Instituto de Ciências Naturais, Humanas e Sociais, Sinop-MT, Brasil, CEP: 78550-728
3 Insituto de Defesa Agropecuária do Estado de Mato Grosso, Sinop-MT, Brasil ,CEP: 78550-360.
(*E-mail: carmenwobeto2014@gmail.com)
ABSTRACT
In this study, we investigated the in vitro activity against Fusarium proliferatum, chemical characteristics, and antioxidant potential of eight propolis ethanolic extracts (PEE) from Brazil-six from the northern region of the state of Mato Grosso (PEE-1 to PEE-4, PEE-6, and PEE-7), one from the state of Bahia (PEE-5), and the green propolis from Minas Gerais state (PEE-8). The percentage of mycelial growth inhibition (PMGI), and macroconidia and microconidia sporulation of Fusarium proliferatum of these PEEs were evaluated and compared with that of the control (ethanol 70%). The 2% concentrations of PEE-4 to PEE-8 showed significant PMGI in relation to the control. PEE-8 at 2% stood out with the highest PMGI of Fusarium proliferatum and highest antioxidant potential. Among the PEEs that exhibited a significant antifungal action, PEE-8 and PEE-4 presented the highest total flavonoid content and PEE-6 stood out with the highest total phenolic content. Except PEE-1, all the extracts tested showed a reduction of more than 70% in the sporulation of macroconidia PEE-4 and PEE-8 presented predominance of quercetin and kaempferol flavonoids and PEE-6 of caffeic acid. Therefore, the presence of these antioxidant compounds may have contributed to the antifungal activity of these extracts.
Keywords: antifungal, alternative control, phenolics, antioxidant potential.
RESUMO
Neste estudo foi investigada a atividade no controle in vitro de Fusarium proliferatum, a composição quÃmica e a atividade antioxidante de oito extratos etanólicos de própolis (EEP) produzidos no Brasil, seis da região norte do estado de Mato Grosso, (EEP-1 até EEP-4, EEP-6 e EEP-7); um do estado da Bahia, Brasil (EEP-5) e a própolis verde do estado de Minas Gerais, Brasil (EEP-8). Foi avaliado o efeito dos EEP na percentagem de inibição do crescimento micelial (PIC) e na esporulação de macroconÃdios e microconÃdios de Fusarium proliferatum e comparado com o controle (etanol 70%). Os extratos EEP-4 até EEP-8 a 2% apresentaram PIC significativos em relação ao controle. O EEP-8 a 2% se destacou com o mais alto PIC de Fusarium proliferatum e o maior potencial antioxidante. De entre os EEP que apresentaram ação antifúngica significativa os EEP-8 e EEP-4 apresentaram os maiores teores de flavonoides totais e o EEP-6 destacou-se com os teores superiores de fenólicos totais. Todos os EEP investigados, exceto o EEP1, provocaram redução de mais de 70% na esporulação de macroconÃdios. Os EEP-4 e EEP-8 apresentaram predominância dos flavonóides quercitina e campferol e o EEP-6 do ácido cafeico. Estes compostos antioxidantes podem ter contribuÃdo para a atividade antifúngica dos extratos.
Palavras-chave: antifúngico, controle alternativo, fenólicos, potencial antioxidante.
INTRODUCTION
Propolis is a gummy product produced by Apis mellifera bees during plant resin harvesting and is a form of beehive protection, used to seal cracks or in embalming invaders. The chemical composition of propolis is complex and varies according to botanical origin, seasonality, and geographic region. Therefore, it is studied in different regions of Brazil and also in other countries (Park et al., 2002; Sousa et al., 2007; Dezmirean et al., 2017; Martini et al., 2017; Regueira Neto et al., 2017).
There are a few studies on propolis from the northern region of Mato Grosso. Soares and Galbiati (2011) evaluated the chemical and sensory composition of propolis from two cities of these regions, ColÃder and Nova Santa Helena, but they did not evaluate the quantitative and qualitative composition of flavonoids, the main bioactive compounds of the product.
Studies on the antibiotic properties of different types of propolis have been receiving prominence in Agrarian Sciences. According to Marini et al. (2012), phytopathogenic bacteria such as Agrobacterium tumefaciens, Xanthomonas axonopodis pv. phaseoli, and Erwinia chrysanthemi are sensitive to the antibiotic activity of propolis.
However, in relation to the antifungal activity on phytopathogens, the studies do not cover all genera of fungi and there are no studies on the effect of propolis against Fusarium proliferatum (Tripathi and Dubey, 2004; Quiroga et al., 2006; Herrera et al., 2010; Pineda et al. 2010; Curifuta et al., 2012; Pereira et al., 2017).
The fungi of the Fusarium genus are causal agents of diseases in several plant species causing damage to seedlings, roots, fruits, stem, spike, and even grains, where they are responsible for the production of mycotoxins. In scientific reports, nine Fusarium species associated with fruit rot in banana were described: F. proliferatum, F. oxysporum, F. verticillioides, F. sacchari, F. semitectum, F. equiseti, F. concentricum, F. camptoceras, F. solani (John et al., 1996; Anthony et al., 2004; Indrakeerthi and Adikaram, 2011; Ewané et al., 2012; Zeng et al., 2013). In addition, the occurrence of Fusarium oxysporum f. sp. cubense, the causal agent of Panama disease, was also reported (Pereira et al., 2005).
Crown rot disease in banana is caused by Lasiodiplodia theobromae, Fusarium proliferatum, and Colletotrichum musae (Anthony et al., 2004; Indrakeerthi and Adikaram, 2011). This disease results in reduction in shelf life and quantity and quality of the fruit produced, affecting both domestic market and exports (Anthony et al., 2004). In the case of F. proliferatum, this phytopathogen interferes in fruit quality for industrial use and produces mycotoxins, which are harmful to human and animal health; therefore, it is necessary to control this fungus for food safety (Li et al., 2017).
In this study, we investigated the chemical composition of propolis of the northern region of Mato Grosso, the green propolis of Minas Gerais, and a sample of brown propolis from Bahia and the in vitro effect of its ethanolic extracts (PEE) on the control of Fusarium proliferatum isolated from symptomatic fruits of banana.
MATERIAL AND METHODS
Sample collection and extract preparation
In August 2017, eight samples of propolis (P) were collected from Porto dos Gaúchos city, Mato Grosso state (P1, P2, and P3); Sorriso city, Mato Grosso state (P4); Bahia state (P5); Querência city, Mato Grosso state (P6); Sinop city, Mato Grosso state (P7); and green propolis from the state of Minas Gerais (P8).
The methodology described by Woisky and Salatino (1998), with some modifications, was employed for preparation of propolis ethanolic extracts (PEE): 20% propolis in 70% ethanol was used under 15 days of maceration, with three daily manual shaking. The extracts were filtered using qualitative filter paper, identified as PEE-1 to PEE-8 and kept under refrigeration in an amber bottle until the biological tests and chemical analyses were carried out.
Assay of propolis effect on Fusarium proliferatum control
The in vitro assays were performed at the Laboratory of Microbiology/Phytopathology of the Federal University of Mato Grosso, Campus of Sinop, with Fusarium proliferatum isolated from banana.
The tests were performed in five replicates using PEE at two concentrations (1,6 and 2,0%) and 70% ethanol (ET 70%) as control. The PEEs were filtered for sterilization on Millipore membranes (0,45 µm) under reduced pressure.
For mycelial growth tests, culture medium potato dextrose agar (PDA, Neogen Co.; Lansing, MI, USA) was employed. The medium was placed on Petri dishes and 0.10 mL of PEE (1,6 and 2,0%) were distributed on the surface of the culture medium. Thereafter, a 7-mm-diameter disc containing mycelium of 20-day-old F. proliferatum was placed in the center of each dish. The dishes were covered with plastic film and incubated at 25° C for 7 days in the dark. Diameter measurement of the colonies was performed every 24 hours until one of the treatments reached the total diameter of the Petri dish. The mycelial growth rate index (MGI) and percentage of mycelial growth inhibition (PMGI) were calculated (Franzener et al., 2006).
From each colony used during the mycelial growth bioassay, spore suspensions were prepared by the addition of 10 mL of distilled water on the dishes, scraping, and gauze filtration, followed by the counting of F. proliferatum macro and microconidia using a Neubauer chamber under an optical microscope (magnification of 400 times).
Physicochemical analysis
The chemical analyses were carried out at the Food Technology Laboratory of the Federal University of Mato Grosso (UFMT)/Sinop Campus, whereas chromatographic analyses were carried out at the Integrated Chemistry Research Laboratory (LIPEQ)/ UFMT / Sinop Campus.
The content of ash and soluble solids in methanol in crude propolis was determined by gravimetric methods (Instituto Adolfo Lutz, 2008). Phenolic compounds and total flavonoids in extracts were determined by spectrophotometric methods using Folin-Ciocalteau and aluminum chloride as reagents and gallic acid and quercetin as standard, respectively (Woisky and Salatino, 1998).
Antioxidant activity was determined by sequestration of the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical with a reading at 517 nm, using butylated hydroxytoluene butyl-hydroxy-toluene (BHT) as a sequester control and employing the following formula to calculate the antioxidant activity: Antioxidant Activity (%) = 100 - {[(Control Absorbance - Sample Absorbance à 100)] / Control Absorbance} (Carpes et al., 2009).
The PEE that showed the highest antifungal effect and antioxidant potential were investigated for phenolic acid and flavonoid content by high performance liquid chromatography (HPLC), according to the methodology described by Barbari‘c et al. (2011), with modifications. Varian Modular Analytical HPLC Systems, equipped with a UV spectrophotometric detector at 290 nm, was employed. C18 reverse phase chromatography column (Agela, 4,6 mm à 250 mm and 5 μm particle diameter) was used with two mobile phases (A and B): water / methanol / acetic acid (93:5:2) (A) and water / methanol / acetic acid (3:95:2) (B). Elution was performed at a flow rate of 1 mL min-1, using the following gradient expressed in time (min) by percentage of B (t /min, % B): (0, 20), (20, 40) (30, 52), (50, 60), (70, 80), (80, 20). The PEE were rotated and solubilized in HPLC grade methanol (10 mg/mL) and a 20 μL aliquot was injected into the chromatographic system. For the identification and quantification of phenolic acids and flavonoids, standard curves were constructed using the following reference substances: gallic acid, caffeic acid, Ï-cumaric acid, ferric acid, quercetin, apigenin, and kaempferol.
Statistical analysis
The experiment was performed in a completely randomized design (CRD), with three replicates for the chemical analyses and five replicates for the bioassays. The averages were compared by the Scott Knott test (p < 0,05) using Sisvar 5.8 software (Ferreira, 2011).
RESULTS AND DISCUSSION
The green propolis extract (PEE-8) at 2% stood out due to the highest PMGI of Fusarium proliferatum (Figure 1) and the most pronounced antioxidant potential (Table 1). The extracts at 2% of PEE-4 to PEE-7 presented lower values of PMGI than PEE-8, and differed from the control (ethanol 70%), whereas those of PEE-1 to PEE-3 did not differ from the control (Figure 1).
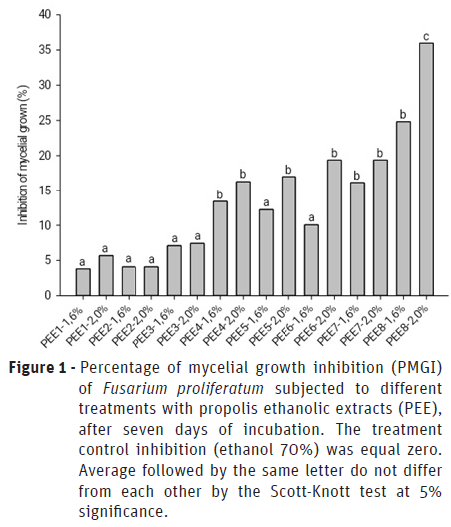
Among the PEE that presented significant antifungal action (Figure 1), PEE-8 and PEE-4 presented the highest levels of total flavonoids and PEE-6 presented significantly higher total phenolic content (Table 1). These observations suggest a correlation between the antifungal effect and the levels of these compounds (Chaillou and Nazareno, 2009; Yang et al., 2011; Curifuta et al., 2012; Waller et al., 2017).
Martini et al. (2017) also verified the antifungal effect of the green propolis against Lasiodiplodia theobromae in vitro. The propolis collected in the summer and in the winter showed high antifungal potential and high content of total phenolics and total flavonoids, which varied from 0,1 to 12,8 g 100 g-1 and from 0,2 to 7,6 g 100 g-1, respectively.
However, there was no correlation between phenolic levels and antifungal activity in PEE-5 and PEE-7, but the ash levels of both did not differ from that of PEE-8, which stood out by the higher levels of this parameter (Table 1). In addition, the solid content of PEE-7 in methanol was inferior only to PEE-8, which suggests that the antifungal effect can be related to other chemical compounds apart from phenolics or to the synergistic effect among these, since propolis is a complex balsamic mixture that presents more than 300 different substances (Sforcin and Bankova, 2011). Buchta et al. (2011) reported a partial correlation only between the levels of flavonoids in propolis and their antifungal action in vitro.
The extracts PEE-4, PEE-6, and PEE-8 at 1,6 and 2,0% caused a reduction of 71% and 76%, respectively, in the sporulation of F. proliferatum macroconidia and a mean reduction of 32% in the sporulation of microconidia for both the concentrations tested (Figure 2). Marini et al. (2012) observed a reduction of 47% in the germination of Phakopsora euvitis spores employing PEE at 0,5%. The differences observed may be due to different botanical origins of propolis and behavior variation of the fungi investigated (Park et al., 2002; Castro et al., 2007; Baribari’c et al., 2011; Curifuta et al., 2012).
PEE-4, PEE-6, and PEE-8 were selected for the investigation of phenolic acids and flavonoids by HPLC due to their outstanding effects on the control of Fusarium proliferatum and high antioxidant potential. The levels of the phenolic acids and flavonoids investigated in the mentioned extracts are shown in Table 2.
PEE-4 and PEE-8 presented considerable levels of flavonoids (Tables 1 and 2), with predominance of quercitin and kaempferol. In PEE-6, the highest phenolic acid content was observed, with caffeic acid being predominant. These antioxidant compounds possibly contributed to the antifungal activity of extracts (Yang et al., 2011; Regueira Neto et al., 2017).
The quercitin and kaempferol levels observed in this study corroborate those reported by Chailou and Nazareno (2009), who evaluated propolis from 30 different locations in Santiago del Estero, Argentina, where they found a wide range of levels from 0,8 to 47,2 mg g-1 and from 0,02 to 20,3 mg g-1, for quercin and kaempferol, respectively.
The antifungal effect of propolis may be due to certain flavonoids or the synergistic effect of these compounds. Further, their action can be differentiated on each pathosystem. Quiroga et al. (2006) reported a higher percentage inhibition of Fusarium oxysporum growth by the use of solutions (1,16 mg ml-1) of the flavonoids pinocembrin (63,9%) and galangin (75,5%) than that of the extract of partially purified propolis (55,6%).
CONCLUSIONS
The green propolis has a strong fungitoxic action against Fusarium proliferatum. Among the propolis of the northern region of Mato Grosso in two were observed high phenolics levels and action against the same fungus. Therefore, this regional beekeeping chain has potential for propolis production for use in agriculture.
REFERENCES
Anthony, S.; Abeywickrama, K.; Dayananda R.; Wijeratman, S.W. & Arambewela, L. (2004) - Fungal pathogens associated with banana fruit in Sri-Lanka, and their treatment with essential oils. Mycopathologia, vol. 157, n. 1, p. 91â97. https://doi.org/10.1023/B:MYCO.0000012226.95628.99 [ Links ]
Barbari’c, M.; MikovÃc, K.; BojÃc, M.; Loncar, M.B.; SmolcÃc-Bubalo, A.; Debeljaka, Z. & MedÃc-SarÃc, M. (2011) - Chemical composition of the ethanolic propolis extracts and its effect on HeLa cells. Journal of Ethnopharmacology, vol. 135, n. 3, p. 772-778. http://dx.doi.org/10.1016/j.jep.2011.04.015
Buchta, V.; Äerný, J. & Opletalová, V. (2011) - In vitro antifungal activity of propolis samples of Czech and Slovak origin. Central European Journal of Biology, vol. 6, n. 2, p. 160-166. http://dx.doi.org/10.2478/s11535-010-0112-3 [ Links ]
Carpes, S.T.; Mourão, G.B.; Alencar, S.M de & Masson, M.L. (2009) - Chemical composition and free radical scavenging activity of Apis mellifera bee pollen from Southern Brazil. Brazilian Journal of Food Technology, vol. 12, n. 3, p. 220-229. http://dx.doi.org/10.4260/BJFT2009800900016 [ Links ]
Castro, M.L.; Cury, J.A.; Rosalen, P.L.; Alencar, S.M.; Ikegaki, M.; Duarte, S. & Koo, H. (2007) - Própolis do sudeste e nordeste do Brasil: influência da sazonalidade na atividade antibacteriana e composição fenólica. QuÃmica Nova, vol. 30, n. 7, p. 151-1516. http://dx.doi.org/10.1590/S0100-40422007000700003 [ Links ]
Chaillou, L.L. & Nazareno, M.A. (2009) - Bioactivity of propolis from Santiago del Estero, Argentina, related to their chemical composition. LWT - Food Science and Technology, vol. 42, n. 8, p. 1422-1427. https://doi.org/10.1016/j.lwt.2009.03.002 [ Links ]
Curifuta, M.; Vidal, J; Sánchez-Venegas, J.; Contreras, A.; Salazar, L.A. & Alvear, M. (2012) - The in vitro antifungal evaluation of a commercial extract of Chilean propolis against six fungi of agricultural importance Ciencia e Investigación Agraria, vol. 39, n. 2, p. 347-359. http://dx.doi.org/10.4067/S0718-16202012000200011 [ Links ]
Dezmirean, D.S.; Marghitas, L.A.; Chirila, F.; Copaciu, F.; Simonca, V.; Bobis, O. & Erlen, S. (2017) - Influence of geographic origin, plant source and polyphenolic substances on antimicrobial properties of propolis against human and honey bee pathogens. Journal of Apicultural Research, vol. 56, n. 5, p. 588-597. https://doi.org/10.1080/00218839.2017.1356205 [ Links ]
Ewané, C.A.; Lepoivre, P.; Bellaire, L. de L. de & Lassois L. (2012) - Involvement of phenolic compounds in the susceptibility of bananas to crown rot. A review. Biotechnology, Agronomy, Society and Environment, vol. 16, n. 3, p. 393-404. [ Links ]
Ferreira, D.F. (2011) - Sisvar: a computer statistical analysis system. Ciência e Agrotecologia, vol. 35, n. 6, p. 1039-1042. http://dx.doi.org/10.1590/S1413-70542011000600001 [ Links ]
Franzener, G.; Stangarlin, J.R.; Czepak, M.P.; Schwa-Nestrada, K.R.F. & Cruz, M.E.S. (2006) - Atividade antibacteriana, antifúngica e indutora de fitoalexinas de hidrolatos de plantas medicinais. Semina - Ciências Agrárias, vol. 28, n. 1, p. 29-38. [ Links ]
Herrera, C.; Alvear, M.; Barrientos, L.; Montenegro, G. & Salazar, L.A. (2010) - The antifungal effect of six commercial extracts of Chilean propolis on Candida spp. Ciencia e Investigación Agraria, vol. 37, n. 1, p. 75-84. http://dx.doi.org/10.4067/S0718-16202010000100007 [ Links ]
Indrakeerthi, S.R.P. & Adikaram, N.K.B. (2011) - Control of crown rot of banana using Carica papaya latex. Journal of the National Science Foundation of Sri Lanka, vol. 39, n.2, p. 155-162. http://dx.doi.org/10.4038/jnsfsr.v39i2.3176 [ Links ]
Instituto Adolfo Lutz (2008) - Métodos fÃsico-quÃmicos para análise de alimentos. 4ª ed., 1a ed. digital. São Paulo, Editora do Instituto Adolfo Lutz, 1020 p. [cit. 2017.06.12]. <http://www.ial.sp.gov.br/resources/editorinplace/ial/2016_3_19/analisedealimentosial_2008.pdf> [ Links ].
John C.D.; Dov, P. & Peter, J. (1996) - Fruit Diseases. In: Ploetz, R. (Ed.) - The mango. Wallingford, UK., CAB International, p. 257-280. [ Links ]
Li T.; Wu, Q.; Wang, Y.; John, A.; Qu, H.; Gong, L.; Duan, X.; Zhu, H.; Yun, Z. & Jiang, Y. (2017) - Application of Proteomics for the Investigation of the Effect of Initial pH on Pathogenic Mechanisms of Fusarium proliferatum on Banana Fruit. Frontiers in Microbiology, vol. 8, art. 2327. https://doi.org/10.3389/fmicb.2017.02327 [ Links ]
Marini, D.; Mensch, R.; Freiberger, M.B.; Dartora, J.; Franzener, G.; Gracia, R.C. & Stangarlin, J.R. (2012) - Efeito antifúngico de extratos alcoólicos de própolis sobre patógenos da videira. Arquivos do Instituto Biológico, vol. 79, n. 2, p. 305-308. http://dx.doi.org/10.1590/S1808-16572012000200023 [ Links ]
Martini, D.; Barbosa, G.F.; Matias, R.; Marques Filho, W.C. & Garcia, N. Z. T. (2017) -Seasonality on the antifungal potential of green propolis collected in Campo Grande â MS, Brazil. Ciência Rural, vol. 47, n.3, art. e20160312. http://dx.doi.org/10.1590/0103-8478cr20160312 [ Links ]
Park, Y.K.; Alencar, S.M.; Scamparini, A.R.P. & Aguiar, C.L. (2002) - Própolis produzida no sul do Brasil, Argentina e Uruguai: evidências fitoquÃmicas de sua origem vegetal. Ciência Rural, vol. 32, n. 6, p. 997-1003. http://dx.doi.org/10.1590/S0103-84782002000600013 [ Links ]
Pereira, J.C.R.; Pereira, J.R.; Castro, M.E.A. & Gasparotto, L. (2005) - Ocorrência do mal-do-panamá em bananeira do subgrupo Figo, em Piau, Minas Gerais. Fitopatologia Brasileira, vol. 30, n. 5, p. 554. http://dx.doi.org/10.1590/S0100-41582005000500022 [ Links ]
Pereira, C.S.; Rempel, D.; Sinhorin, A.P.; Fernandes, H. & Fiorini, I.V.A. (2017) - Aplicação de extrato etanólico de própolis em doenças da cultura da soja. Revista de Ciências Agrárias, vol. 40, n. 4, p. 854-862. http://dx.doi.org/10.19084/RCA17029 [ Links ]
Pineda, J.; Principal, J.; Barrios, C.; Milla, D.; Solano, Y. & Gil, E. (2010) - Propiedad fungistática in vitro de propóleos sobre tres aislamientos de Colletotrichum gloeosporioides. Zootecnia Tropical, vol. 28, n. 1, p. 83-91. [ Links ]
Quiroga, E.N.; Sampietro, D.A.; Soberon, J.R.; Sgariglia, M.A. & Vattuone, M.A. (2006) - Propolis from the northwest of Argentina as a source of antifungal principles. Journal of Applied Microbiology, vol. 101, n. 1, p. 103â110. https://doi.org/10.1111/j.1365-2672.2006.02904.x [ Links ]
Regueira Neto, M.S.; Tintino, S.R.; Silva, A.R.P. da; Costa, M. do S.; Boligon, A.A.; Matias, E.F.F.; Balbino, V. de Q.; Menezes, I.R.A. & Coutinho, H.D.M. (2017) - Seasonal variation of Brazilian red propolis: Antibacterial activity, synergistic effect and phytochemical screening. Food and Chemical Toxicology, vol. 107, part B, p. 572-580. https://doi.org/10.1016/j.fct.2017.03.052 [ Links ]
Sforcin, J.M. & Bankova, V. (2011) - Propolis: Is there a potential for the development of new drugs? Journal of Ethnopharmacology, vol. 133, n. 2, p. 253-260. https://doi.org/10.1016/j.jep.2010.10.032 [ Links ]
Soares, E.M. & Galbiati, C. (2011) - Caracterização fÃsico-quÃmica da própolis de Apis mellifera (L.) em apiários de Colider e Nova Santa Helena, território do portal da Amazônia, Mato Grosso. In: Santos, J.E.; Galbiati, C. & Moschini, L.E. (Eds.) - Gestão e Educação Ambiental: água, biodiversidade e cultura. São Carlos, RiMa, p. 67-81. [ Links ]
Sousa, J.P.B.; Furtado, N.A.J.C.; Jorge, R., Soares, A.E.E. & Bastos, J.K. (2007) - Perfis fÃsico-quÃmico e cromatográfico de amostras de própolis produzidas nas microrregiões de Franca (SP) e Passos (MG). Revista Brasileira de Farmacognosia vol. 17, n. 1, p. 85-93. [ Links ]
Tripathi, P. & Dubey. N.K. (2004) - Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biology and Technology, vol. 32, n. 3, p. 235-245. https://doi.org/10.1016/j.postharvbio.2003.11.005 [ Links ]
Waller, S.B.; Peter, C.M.; Hoffmann, J.F.; Picoli, T.; Osório, L. da G.; Chaves, F.; Zani, J.L.; Faria, R.O. de; Mello, J.R.B. de & Meireles, M.C.A. (2017) - Chemical and cytotoxic analyses of brown Brazilian propolis (Apis melifera) and in vitro activity against itraconazole-resistant Sporothrix brasiliensis. Microbial Pathogenesis, vol. 105, p. 117-121. https://doi.org/10.1016/j.micpath.2017.02.022 [ Links ]
Woisky, R.G. & Salatino, A. (1998) - Analysis of propolis: some parameters and procedures of chemical quality control. Journal of Apicultural Research, vol. 37, n. 2, p. 99-105. https://doi.org/10.1080/00218839.1998.11100961 [ Links ]
Yang, S.Z.; Peng, L.T.; Su, X.J.; Chen, F.; Cheng, Y.J.; Fan, G. & Pan, S.Y. (2011) - Bioassay-guided isolation and identification of antifungal components from propolis against Penicillium italicum. Food Chemistry, vol. 127, n. 1, p. 210â215. https://doi.org/10.1016/j.foodchem.2010.12.011 [ Links ]
Zeng, L.S.; Zhao, Z.H.; Lu, S.; Xi, Z.J.; Li, M.H. & Xi, P.G. (2013) - The Fusarium species isolated from banana and their phylogenetic relationships. Mycosystema, vol. 32, n. 4, p. 617-632. [ Links ]
ACKNOWLEDGMENTS
The authors thank National Council for Scientific and Technological Development (CNPq) for granting a scientific initiation grant.
Received/recebido: 2018.09.07
Aceite/accepted: 2019.03.13














