Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de Ciências Agrárias
Print version ISSN 0871-018X
Rev. de Ciências Agrárias vol.41 no.2 Lisboa June 2018
https://doi.org/10.19084/RCA16142
ARTIGO
Biofertilizer effect of yeast fermented broth on organic tomato seedlings
Efeito biofertilizante de caldo fermentado por levedura em mudas de tomate orgânico
Luiz Gabriel Gemin*, Roberto Datsch, Átila Francisco Mógor and Gilda Mógor
Postgraduate Course on Crop Science, Federal University of Paraná - UFPR, Curitiba, Brazil
(*E-mail: gemin1988@hotmail.com)
ABSTRACT
Looking for the development of products based on natural sources capable of promoting plant growth according to organic production assumptions, the aim of this work was to evaluate the effect of Saccharomyces cerevisiae fermented broth (SFB) as a biofertilizer on organic tomato seedlings growth and chlorophyll content of leaves, taking into account the content of levorotatory configuration amino acids in SFB. For that reason, were performed foliar applications of aqueous solutions with five concentrations (0,25; 0,50; 0,75; 1,0 and 1,25 mL.L-1) of SFB (complex-aid® - Alltech®) and a control with application of distilled water. The SFB showed remarkable efficiency in promoting tomato seedlings growth, increasing leaves chlorophyll content, increasing leaves and stems growth, roots volume and altering its diameter partitioning, improving the rate of thinner roots at 0,75 mL L-1 concentration. These are initial results for further investigations about how SFB acts on plant metabolism, related to L-amino acids or other possible bioactive compounds released by Saccharomyces cerevisiae.
Keywords: Saccharomyces cerevisiae, plant growth, L- amino acids, chlorophyll.
RESUMO
Buscando o desenvolvimento com base em fontes naturais capazes de promover o crescimento de plantas de acordo com os pressupostos da produção orgânica, o objetivo deste estudo foi avaliar o efeito do caldo fermentado de Saccharomyces cerevisiae (SFB) como biofertilizante no crescimento de mudas tomate em sistema orgânico e quantificar o conteúdo de clorofila nas folhas, tendo em conta que o SFB apresenta aminoácidos com configuração levógira. Por esse motivo, as aplicações foliares de soluções aquosas foram realizadas com cinco concentrações (0,25, 0,50, 0,75, 1,0 e 1,25 ml.l-1) de SFB (complex-Aid ® - Alltech®) e uma testemunha com aplicação de água destilada. O SFB mostrou notável eficácia na promoção do crescimento das mudas de tomate, aumentando o teor de clorofila das folhas, no aumento do crescimento de folha e caule, no volume das raízes estratificado por diâmetro, melhorando a taxa de raízes mais finas em 0,75 ml. L-1 de concentração. Estes são os primeiros resultados de nova investigação sobre a utilização de SFB sobre o metabolismo da planta em conjunto com L-aminoácidos e outros compostos bioativos possíveis divulgados pela Saccharomyces cerevisiae.
Palavras-chave: Saccharomyces cerevisiae, crescimento vegetal, L- aminoácidos, clorofila.
INTRODUCTION
From the central part of the Andean region in South America, and after its domestication in Mexico, the cultivation of tomato (Solanum lycopersicum) has spread to the world. In Brazil, the introduction of tomato influenced European immigrants in the nineteenth century. Nowadays, it is one of the most important vegetable crops in plantation area, production, and value (Alvarenga, 2013).
The productive efficiency of traditional tomato production should ensure high yields. So, it becomes necessary to use high quantities of synthetic inputs to increase the production, thereby causing an invariable decrease in the sustainability of production system that can affect the soil, water, and the health of growers and consumers (Silva et al., 2007).
Despite this, the organic farming system has shown a rapid growth in Brazil in in recent years, driven by the demand of the population for healthy food, increasing production without the use of synthetic fertilizers and pesticides (Kiss, 2004). Additionally, organic production is an excellent business opportunity and undoubtedly a great challenge to producers, who need information about cultivars, pests and diseases control (Khatounian, 2001), besides natural sources that can promote plant growth. Therefore, the great challenge is to develop or to adapt tomato production technologies that could enable growers to achieve greater economic returns concomitant with the production system sustainability.
The practice of sustainable agriculture, such as organic system enhances environmental conservation, produces free-chemical contamination food and upper biological and commercial value. Additionally, it leads to an increase of hand labor, keeping the men at the field allied to more satisfactory economic returns. However, despite the increasing expansion involving organic agriculture, competitiveness and sustainability of production units still rely heavily on knowledge and technology generation on a scientific basis.
The development of new environmentally safer technologies is an international trend and urgent for growing vegetables, where chemicals are usually used in indiscriminately. Among these technologies, the use of products from fermentation processes, as biofertilizers, stand out by showing recent results (Bettoni et al., 2014; Kaseker et al., 2014; Röder, 2014).
As a result, promoting the development of sustainable systems in tomato production by exploiting the full potential of growing and contributing to the increase in the welfare of growers and consumers are the strategies to be followed. In this context, organic production gains importance (Sediyama, 2014), and biofertilizers could be an important tool, acting in the improvement of the performance of production systems by stimulating plant metabolism, as observed on the activity of roots growth (Bezerra, 2007).
The use of biofertilizers is a technique set by the Brazilian regulation of organic production (Brasil, 2003). This regulation defines biorfertilizers as products that contain active compounds or biological agents able to act directly or indirectly on all or part of cultivated plants, improving the production system performance and making unnecessary the use of the substance of disallowed chemicals within organic system regulation (Brasil, 2011).
Among the products with biofertilizer effect potential, some can be obtained through a fermentation process using sugar cane broth, an efficient carbon source for the growth of microorganisms. According to Santos and Akiba (1996), these fermented broths could be planted metabolic activators and are composed by proteins, enzymes, vitamins, and amino acids produced and released by microorganisms during fermentation processes, acting as stimulators of plant growth.
All plants produce their AA. Studies have demonstrated that the exogenous supply of AA may result in beneficial effects, such as significant gains in various processes of growth and development of plants. Such fact has been confirmed by providing a solution containing the L-AA to plants, which is absorbed and incorporated to the plant metabolism as a precursor of the aminolevulinic acid on chlorophyll synthesis (Beale et al., 1975), or improving the nitrate reductase (EC 1.7.99.4) activity and a consequent plant growth related to nitrogen metabolism (Rӧder, 2014).
Mógor et al. (2008) demonstrated significant results of the application of isolated AA or in association with Ascophyllum nodosum extract on common beans (Phaseolus vulgaris L.), which promoted a greater early growth of bean plants as well as the increase in grain production. In a recent work, the foliar application of a sugar cane fermented broth containing L-glutamic acid in cabbage seedlings showed significant results, increasing chlorophyll levels and roots volume (Rӧder et al., 2015).
Consequently, it can be inferred that L-AA derived from fermentation processes are potential active components of biofertilizers, since they are considered metabolic activators (Bezerra et al., 2007), and due to their capability of acting as plant growth stimulators.
For being a yeast used since ancient times in various kind of fermentations as a leavening agent (bread, beer, production of ethyl alcohol), Saccharomyces cerevisiae, whose metabolism is known the best, is the eukaryote organism studied the most (Yamada et al., 2003). The S. cerevisiae was widely used as a model organism to elucidate the bionsynthesis pathways of amino acids (Braus, 1991; Cooper et al., 2010), whose high amino acid content has been reported since Watson (1976).
The autolysis of yeast cell wall by the present enzymes or acids releases the cell content (Dawson, 2002), including the L-AA (Watson, 1976). As a result, their fermented broth could be a source of L-AA composition for sustainable agricultural use.
Regarding tomato crops, the success of the production is first achieved by the formation of high quality seedlings, showing homogeneity and adequate growth of roots and stems for transplanting (de Paiva, 2011). According to Maggioni et al. (2014), the seedling production practice interferes directly in the final performance of plants and gives advantages such as economy of seeds, less use of pesticides and increased fixation field after transplantation.
Therefore, the objective of this study was to evaluate the likely biofertilizer effects of foliar applications of the S. cerevisiae fermented broth on tomato seedlings growth under organic system.
MATERIAL AND METHODS
The experiment was conducted in a protected environment (polyethylene film covered nursery) at the organic garden of the Federal University of Paraná, Brazil, at coordinates 25°23'30'' S and 49°07'30'' W, 920 m altitude. The climate in the region is temperate humid (Mesothermal) according to Köppens classification. The tomato cultivar 'Santa Cruz' (Topseed®) was used, as it is commonly used among organic farmers, planted on polystyrene trays with 200 cells, filled with commercial substrate (Bioplant®). Sowing was held on August 12, 2014. The treatments with four replications were composed of 100 cells each, distributed in a completely randomized design and consisted of foliar applications of aqueous solutions with five concentrations (0.25; 0.50; 0.75; 1.0 and 1.25 mL.L-1) of a S. cerevisiae fermented broth (complex-aid® - Alltech®) and a control with application of distilled water. The foliar sprays were performed using a CO2 pressurized sprayer at constant pressure (45 lib.pol-1) at the amount of 100 mL per tray. Three sprayings were carried out, one each week, starting at 15 days after seeding when the seedlings showed the first true leaves. At 36 days after seeding, the seedlings were randomly collected from each repetition, totaling 28 per treatment.
To identify biofertilizer effect on the promoting of seedling growth the following biometric variables were measured: leaf area, stem volume, root volume and root diameter averages separated in four classes of means (0.5-0.99 mm; 1.0-1.49 mm; 1.5-1.99 mm; 2.0-2.5mm). After carefully washing over a sieve to avoid losing parts of the roots, the seedlings were analyzed using the software WinRhizo® (Regent Instruments Inc. 2013, Canada) coupled to a LA1600 3D Scanner.
Regarding determination of the possible effect of S. cerevisiae fermented broth (SFB), on chlorophyll synthesis, the leaf pigments (chlorophyll a, chlorophyll b, total chlorophyll and carotenoids) were determined according to the method described by Lichtenthaler (1987) with the following modifications: in a microtube, 0.3 g of macerated plant material and 1.8 mL of 80% acetone were added in distilled H2O with 0.1% CaCO3 (w/v); vortex agitation was performed at maximum speed for 10 seconds, at four times for each sample in a three-minute interval between agitations. The solution was centrifuged at 9335 g or 10,000 rpm for two seconds. For the readings at 663 and 647 nm on spectrophotometer, the supernatant was used as well as to obtain the mean values of the formula described by Lichtenthaler and Buschmann (2001).
The data were tested for homogeneity of variances using the Bartlett's test and then analyzed by ANOVA at 5% probability. When significant, the growth and foliar pigments data were submitted to regression analysis. Diameter means of the roots were compared using the test of Duncan (p<0.05). The software Assistat® beta 7.7 was also used (Silva and Azevedo, 2009).
RESULTS
Chlorophyll content in the leaves of the tomato seedlings was affected by SFB treatments. As the SFB concentration increased, total content of chlorophyll increased linearly with 46% of increment at 1 mL.L-1. These results indicate that SFB sprayed over leaves of tomato seedlings was taken up and played a role in chlorophyll synthesis, possible related to their L-AA content. The carotenoids content was not affected by treatments (Figure 1).
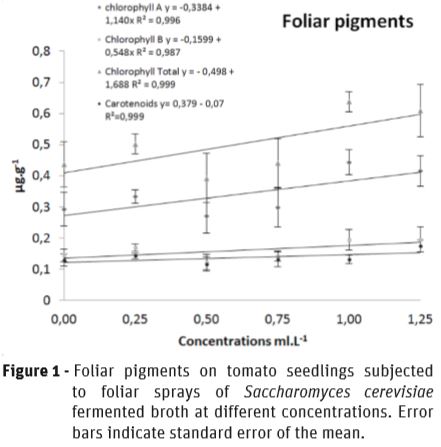
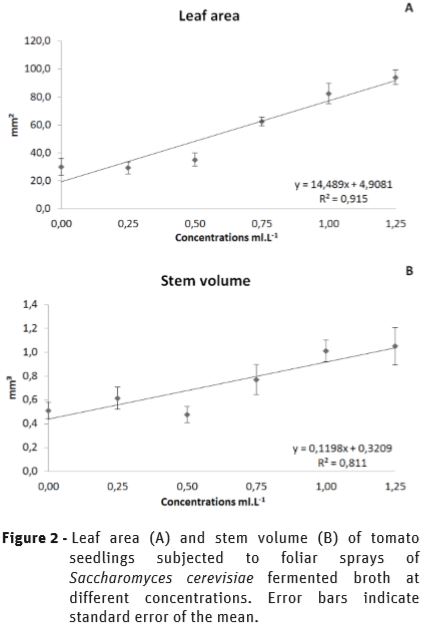
The SFB treatments showed a remarkable effect on growth promotion on leaves and stems of tomato seedlings, showing a linear response to the increases in the concentration of the spray solution, as it was well observed as chlorophyll content was incremented (Figure 1). The leaf area increased by 213.2% (Figure 2A), and the volume of stems by 105.87% (Figure 2B), indicating a significant biofertilizer effect by SFB.
The volume of the roots of tomato seedlings was also affected by SFB treatments, showing a polynomial response to the increase in the concentration (Figure 3). At 1 mL.L-1, the volumes of the roots were 53% higher than the control, yet showing a reduction as the concentration increased.

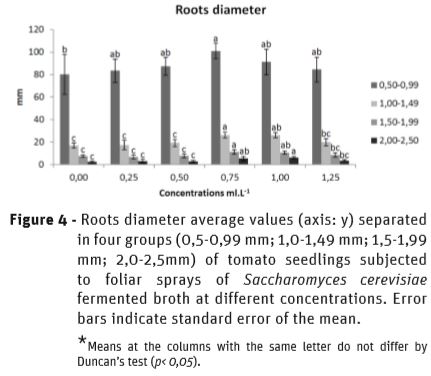
Figure 4 shows the effect of SFB on root diameter partitioning, in which the mean values of the diameter were separated into four groups: 0.5-0.99 mm; 1.0-1.49 mm; 1.5-1.99 mm and 2.0-2.5mm. At 0.75 mL L-1, all diameter groups showed the highest means in comparison to the control, as the higher means were already compared to the lower concentrations (0.25; 0.5 mL L-1) in the 2.0- 2.5mm group at 1 mL L-1. However, at 1.25 mL L-1, a reduction was found in the diameter of the roots, corroborating to the data on the volume of the roots, pointing to the change in the translocation of photoassimilates from the leaves to the roots.
DISCUSSION
Beale et al. (1975), when using a labeled carbon, showed that the AA L-glutamic acid added to the leaves of the plants could be absorbed and metabolically used for chlorophyll synthesis. The authors show that the sprayed solution overcomes the wax barrier in the leaf cuticle and penetrates the apoplast and subsequently cross the plasmalemma so that the destination reached the symplast. Therefore, it can be inferred that the treatment with SFB and its L -AA content applied to the leaves of tomato seedlings could be absorbed and metabolized.
The growth in chlorophyll (CHL) content in the leaves of the seedlings shows the absorption and the metabolization of SFB. Chlorophylls are the most abundant natural pigments in plants, common to all photosynthetic cells. Chlorophyll a, the most abundant and the most important in this family corresponds to approximately 75% of green pigments found in plants (Taiz and Zeiger, 2009). So, the well-known role of CHL on carbon incorporation to the biomas of the plants could be justified by the linear effect of SFB on the the growth of leaves and stems of tomato seedlings.
By observing the complex interactions between CHL and AA on plant growth, it could be considered that the synthesis of many AA is completed in the chloroplast with some consequences on transamination, the conversion of one AA to each other according the metabolic demands (Srivastava, 2002), indicating not just the SFB effect on the enhancement of CHL to justify the plant growth, but also their possible role on AA metabolism and related pathways: AA is a structural unit of peptides, proteins, and also precursors of the numerous plant regulating substances, such as hormones and polyamines.
According to Kerbauy (2008), the assimilation of AA applied to the leaves increased the enzyme activity involved in the metabolism of nitrogen, which may cause greater growth rates, as found by Röder (2014) with the improvement of the nitrate reductase (EC 1.7.99.4) activity on potato leaves (Solanum tuberosum). This improvement is related to the foliar sprays of sugar cane fermented broth containing L-glutamic acid, with consequent plant growth promotion related to the nitrogen metabolism.
Growth of the roots depends on the capacity of the leaves, as a source, to provide photoassimilates (mainly non-reducing sugars) to the roots, as a sink. As a result, the improvement of CHL content and the leaves expansion may justify the changes in the volume of tomato seedlings roots found in SFB treatments.
When working with Origanum vulgare seedlings treated with L-AA based biofertilizer, Bettoni et al. (2014) found that the excellence in the growth of the roots was related to the growth of leaves and stems, but the highest rate of biofertilizer spray reduced the roots volume, corroborating to the data obtained in this work. Similar results were also obtained by Röder et al. (2015) when using foliar application of a sugar cane fermented broth containing L-AA in cabbage seedlings, which showed significant results in the increase of the levels of chlorophyll and in the volume of roots at intermediate concentrations. A reduction in the growth at the highest concentrations was also observed.
The source and sink relationship between leaves and roots could explain the polynomial response of roots volume as a function of the improvement in SFB concentration since the new leaves expansion (Figure 2A) becomes a sink competing with the roots for non-reducing sugars (Srivastava, 2002).
Lea and Forde (2007) reported that application of small doses of L-AA, specifically L-glutamic acid, elicits a response by the plant in producing secondary roots. The results on the roots diameter partitioning of tomato seedlings indicate that SFB not only improved the volume of the roots but also improved the rate of thinner roots at 0.75 mL L-1 concentration in comparison to the control. The 1.0-2.5 mm roots were improved at 0.75 and at 1 mL L-1. The reduction in the amount of roots at 1.25 mL L-1 corroborates to the volume reduction, which was previously discussed.
According to Brandão (2007), the increases in plant growth resulting from the foliar application of products containing L-AA, when absorbed and metabolized may take part in the synthesis of several compounds, with a direct effect on the plant growth. The use of direct fertilization in plants with L-AA could improve the transformation of nitrate to ammonia, and so to AA with rapid incorporation into the metabolism, contributing to the development and growth process (Röder, 2014).
Given the above, it can be inferred that the application and metabolization of L-AA on SFB may promote plant growth by directly influencing pathways such a CHL synthesis and AA metabolism. However, the physiological responses of plants to the application of L-AA are less discussed in the literature and must be better understood.
According to the Brazilian regulation, biofertilizer is defined as a a product that contains one or more active compounds able to act directly or indirectly all over the plant or in some parts of it (Brasil, 2011). So, according to the results previously discussed, SFB could be considered as a biofertilizer and its L-AA content as the active ingredients. It should be considered that the biochemical changes in plants in response to active ingredients contained in the biofertilizers could serve as indicators of the performance of these products. The changes in CHL content of tomato seedlings confirm the SFB bioactivity.
CONCLUSION
It is concluded that SFB is efficient in promoting the growth of tomato seedlings under the organic system, in which synthetic nutrient sources are avoided. The SFB could be a proper tool to improve the seedlings quality with consequent benefits in the field.
However, these are only the first results, which stimulates further investigations that may lead to a better knowledge on how SFB acts on plant metabolism, related to L-AA or many among other possible bioactive compounds released by Saccharomyces cerevisiae during fermentation and autolysis.
References
Alvarenga, M.A.R. (2013) - Tomate: produção em campo, casa de vegetação e hidroponia. 2a ed. Lavras, Editora Universitária Lavras. 455 p. [ Links ]
Beale, S.; Gough, S.P. & Granick, S. (1975) - Biosynthesis of delta-aminolevulinic acid from the intact carbon skeleton of glutamic acid in Greening Barley. Proceedings of the National Academy of Sciences of the United States of America, vol. 72, n. 7, p. 2719-272. [ Links ]
Bettoni, M.B.; Fabbrin, E.G. dos S.; Procopiuk, M. & Mógor, A.F. (2014) - Crescimento de mudas de orégano submetidas a doses e frequências de aplicação de Ácido L-glutâmico em sistema orgânico. Revista Brasileira de Plantas Medicinais, vol. 16, n. 1, p. 83-88. 2014. http://dx.doi.org/10.1590/S1516-05722014000100012 [ Links ]
Bezerra, P.S.G.; Grangeiro, L.G.; Negreiros, M.Z. & Medeiros, J.F. de (2007) - Utilização de bioestimulante na produção de mudas de alface. Científica, vol. 35, n. 1, p. 46-50. http://dx.doi.org/10.15361/1984-5529.2007v35n1p46+-+50 [ Links ]
Brandão, R. P. (2007) - Importância dos Aminoácidos na agricultura sustentável. Informativo BioSoja, vol. 5, p. 6-8. [ Links ]
Brasil (2003) - Lei n° 10.831, de 23 de dezembro de 2003. Dispõe sobre a agricultura orgânica e dá outras providências. Brasília, 23 de dezembro de 2003. [cit. 2016-02-26]. <http://www.planalto.gov.br/ccivil_03/leis/2003/L10.831.htm>.
Brasil (2011) - Instrução normativa nº 46, de 6 de outubro de 2011. Ministério da agricultura, pecuária e abastecimento [cit. 2016-03-15]. < http://www.agricultura.gov.br/arq_editorfile/Desenvolvimento_Sustentavel/Organicos/Legislacao/Nacional/Instrucao_Normativa_n_0_046_de_06-102011_regulada_pela_IN_17.pdf>.
Braus, G.H. (1991) - Aromatic Amino Acid Biosynthesis in the Yeast Saccharomyces cerevisiae: a Model System for the Regulation of a Eukaryotic Biosynthetic Pathway. Microbiological Reviews, vol.55, n. 3, p. 349-370. [ Links ]
Cooper S.J.; Finney G.L.; Brown S.L.; Nelson S.K.; Hesselberth J.; MacCoss, M.J. & Fields, S. (2010) - High-throughput profiling of amino acids in strains of the Saccharomyces cerevisiae deletion collection. Genome Research, vol. 20, p. 1288-1296. http://dx.doi.org/10.1101/gr.105825.110 [ Links ]
Dawson, K. (2002) - Not just bread and beer: new applications for yeast and yeast products in human health. In: Lyons, T.P. & Jacques, K.A. (Eds.) - Proceedings of the 18th Annual Symposium. Alltech Biotechnology: Nottingham University Press, Nottingham, UK, p. 225-232. [ Links ]
de Paiva, E.P.; Maia, S.S.S.; Cunha, C.S.M.; Coelho, M.F.B. & Silva, F.N. (2011) - Composição do substrato para o desenvolvimento de mudas de manjericão (Ocimum basilicum L.). Revista Caatinga, vol. 24, n. 4, p. 62-67. [ Links ]
Kaseker, J.F.; Bastos, M.C.; Consalter, R. & Mógor, Á.F. (2014) - Alteração do crescimento e dos teores de nutrientes com utilização de fertilizante organomineral em cenoura. Revista Ceres, vol. 61, n. 6, p. 964-969. http://dx.doi.org/10.1590/0034-737X201461060011 [ Links ]
Kerbauy, G. (2008) - Fisiologia vegetal. 2ª ed. Rio de Janeiro, Guanabara Koogan, 452 p. [ Links ]
Khatounian, C.A. (2001) - A reconstrução ecológica da agricultura. Botucatu, Agroecologia. 345 p. [ Links ]
Kiss, J. (2004) - Terra em transe. Globo Rural, n. 223, p. 34-42. [ Links ]
Lea, P.J. & Forde, B.G. (2007) - Glutamate in plants: metabolism, regulation, and signaling. Journal of Experimental Botany, vol. 58, n. 9, p. 2339-2358. http://dx.doi.org/10.1093/jxb/erm121 [ Links ]
Lichtenthaler, H.K. (1987) - Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology, vol. 148, p. 350-382. http://dx.doi.org/10.1016/0076-6879(87)48036-1 [ Links ]
Lichtenthaler, H. & Buschmann, C. (2001) - Chlorophylls and carotenoids: Measurement and characterization by UV–VIS spectroscopy. Current Protocols in Food Analytical Chemistry, p. F4.3.1-F4.3.8. http://dx.doi.org/10.1002/0471142913.faf0403s01 [ Links ]
Maggioni, M.S.; Rosa, C.B.C.J.; Rosa, J.E.J.; Silva, E.F.; Rosa, Y.B.C.J.; Scalon, S.P.Q. & Vasconcelos, A.A. (2014) - Desenvolvimento de mudas de manjericão (Ocimum basilicum L.) em função do recipiente e do tipo e densidade de substratos. Revista Brasileira de Plantas Medicinais, vol. 16, n. 1, p. 10-17. http://dx.doi.org/10.1590/S1516-05722014000100002 [ Links ]
Mógor, A.F.; Ono, E.O.; Rodrigues, J.D. & Mógor, G. (2008) - Aplicação foliar de extrato de alga, ácido L-glutâmico e cálcio em feijoeiro. Scientia Agraria, vol. 9, n. 4, p. 431-437. http://dx.doi.org/10.5380/rsa.v9i4.11710 [ Links ]
Röder, C. (2014) - Efeito da aplicação de um fermentado bacteriano contendo ácido L- glutâmico no crescimento, produtividade e alterações bioquímicas em batata cultivada no sistema orgânico. Tese de Doutorado. Curitiba. Universidade Federal do Paraná. 109 p. [ Links ]
Röder, C.; Mógor, A.F.; Szilagyi-Zecchin, V.J.; Fabbrin, E.G.S. & Gemin, L.G. (2015) - Uso de biofertilizante na produção de mudas de repolho. Revista Ceres, vol. 62, n. 5, p. 421-422. http://dx.doi.org/10.1590/0034-737X201562050012 [ Links ]
Santos, A.C. & Akiba, F. (1996) - Biofertilizantes líquidos: uso correto na agricultura alternativa. Rio de Janeiro, Seropédica: Imprensa Universitária/UFRJ. 35 p. [ Links ]
Sediyama, M.A.N.; Santos, I.C. & Lima, P.C. (2014) - Cultivo de hortaliças no sistema orgânico. Revista Ceres, vol. 61, n. sup., p. 829 -837. http://dx.doi.org/10.1590/0034-737x201461000008 [ Links ]
Silva, F. de A.S. & Azevedo, C.A.V. (2009) - Principal Components Analysis in the Software Assistat-Statistical Attendance. In: World congress on computers in agriculture, 7, Reno-NV-USA: American Society of Agricultural and Biological Engineers. [cit. 2016-09-03]. < http://www.assistat.com> [ Links ].
Silva, A.F.; Pinto, J.M.; França, C.R.R.S.; Fernandes, S.C.; Gomes, T.C. de A.; Silva, M.S.L. da & Matos, A.N.B. (2007) - Preparo e Uso de Biofertilizantes Líquidos. Comunicado Técnico da Embrapa Semi-Árido. [cit. 2016-08-21]. <http://www.infoteca.cnptia.embrapa.br/bitstream/doc/339493/1/COT130.pdf> [ Links ]
Srivastava, L.M. (2002) - Plant growth and development: hormones and environment. San Diego, Academic Press. p. 722. [ Links ]
Taiz, L. & Zeiger, E. (2009) - Fisiologia vegetal. 4ª ed. São Paulo, Artmed, 820 p. [ Links ]
Yamada, E.A., Alvim, I.D., Santucci, M.C.C. & Sgarbieri, V.C. (2003) - Composição centesimal e valor protéico de levedura residual da fermentação etanólica e de seus derivados. Revista de Nutrição, vol. 16, n. 4, p. 423-432. http://dx.doi.org/10.1590/S1415-52732003000400006 [ Links ]
Watson, T.G. (1976) - Amino-acid Pool Composition of Saccharomyces cerevisiae as a Function of Growth Rate and Amino-acid Nitrogen Source. Journal of General Microbiology, vol. 96, p. 263-268. http://dx.doi.org/10.1099/00221287-96-2-263 [ Links ]
Received/recebido: 2016.11.01
Accepted/aceite: 2018.02.19














