Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de Ciências Agrárias
Print version ISSN 0871-018X
Rev. de Ciências Agrárias vol.39 no.1 Lisboa Mar. 2016
https://doi.org/10.19084/RCA15062
ARTIGO
Effect of tannin source and pH on stability of tannin-protein and fibre complexes
Efeito da fonte de taninos e do pH na estabilidade dos complexos taninos-proteína e taninos-fibra
Maria. T.P. Dentinho1* and Rui. J.B. Bessa1,2
1 Unidade de Investigação de Produção e Saúde Animal, Instituto Nacional de Investigação Agrária e Veterinária, I.P. Fonte Boa, 2005-048 Vale de Santarém, Portugal. E-mail: teresa.dentinho@iniav.pt
2 CIISA, Faculdade de Medicina Veterinária, Universidade de Lisboa, Polo Universitário do Alto da Ajuda, 1300-477 Lisboa, Portugal.
ABSTRACT
Quebracho (Schinopsis quebracho-colorado Schlecht) is the most extensively tannin extract explored in order to improve the use of protein in ruminant feed. The rock rose (Cistus ladanifer L.) and the grape seed (Vitis vinifera L.) are alternative sources of condensed tannins (CT). The objective of this study was to evaluate the effect of pH on the stability of complexes formed between the rock rose tannins and grape seed and the protein and fiber of soybean meal and compare the results with those obtained with the quebracho tannin extract. The results obtained show that the rock rose and grape seed tannins, such as quebracho tannins, have the ability to bind to soybean meal protein at pH between 6 and 8, reducing the solubility of nitrogen. These complexes are dissociated at pH 2, releasing the protein. This binding pattern supports the concept of using these tannin sources in ruminant nutrition as additives to protect the protein feed from excessive rumen degradation. However it was observed differences among tannin sources. Rock rose and grape seed tannins seem be more effectives in reducing protein degradation in the rumen than quebracho tannins.
Keywords: Cistus ladanifer, grape seed, pH, quebracho, Tannin-protein complexes
RESUMO
O extrato de taninos de quebracho (Schinopsis quebracho-colorado Schlecht) é o extrato que mais tem sido estudado com o objetivo de melhorar a utilização da proteína dos alimentos para ruminantes. A esteva (Cistus ladanifer L.) e a grainha de uva (Vitis vinifera L.) são fontes alternativas de taninos condensados (CT). Foi objetivo deste estudo avaliar o efeito do pH na estabilidade dos complexos formados entre os taninos da esteva e da grainha de uva e a proteína e a fibra do bagaço de soja e comparar com a estabilidade dos complexos formados com os taninos de quebracho. Os resultados obtidos mostram que os taninos de esteva e da grainha de uva, tal como os de quebracho, têm a capacidade de se ligar à proteína de soja entre pH 6 e 8, reduzindo a solubilidade do azoto. Estes complexos são dissociados a pH 2, libertando a proteína. Este padrão de ligação suporta o conceito de utilizar estas fontes de tanino como aditivos para proteger a proteína dos alimentos da excessiva degradação ruminal. Existem contudo diferenças entre as fontes de tanino utilizadas parecendo ser os taninos de esteva e de grainha de uva mais eficazes na redução da degradação ruminal da proteína que os taninos de quebracho.
Palavas-chave: Cistus ladanifer, complexos tanino-proteína, grainha de uva, pH, quebracho.
Introduction
Tannins are phenolic compounds functionally defined by their capacity to complex macromolecules (proteins and polysaccharides) and metal ions. Complexation with proteins is the basis of many biological effects of tannins (Min et al., 2003).
Traditionally tannins are grouped into two classes, hydrolysable and condensed tannins (CT) or proanthocyanidins. The CT are the most common type of tannins found in forage legumes, trees and shrubs (Barry and McNabb, 1999; Mueller-Harvey, 2006) and they are the most commonly responsible for the range of reactions attributed to the presence of tannins in plants (Porter, 1992).
The CT effects on animal nutrition have been extensively studied and are considered to have both, adverse and beneficial effects on the animal depending on concentration and chemical structure (Makkar, 2003b; Min et al., 2003; Mueller-Harvey, 2006).
Tannin-protein complexes are established by non-covalent bonds (hydrogen and hydrophobic interactions, van der Waals forces) or covalent bonds (Bourvellec and Renard, 2006). The covalent bonds are usually irreversible but the non-covalent bonds can be reversible depending on many factors such as pH, structure and molecular weight of CT and proteins (Hagerman, 1989; Bourvellec and Renard, 2006). The pH-dependence of tannin-protein complexes is particularly interesting in ruminant nutrition, whereas pH variation along the digestive system defines the nutritional behaviour of CT. In ruminants, one of the most important effects of CT intake is associated with their capacity to increase the digestive utilization of dietary protein. This effect is due to CT ability to bind proteins under rumen pH conditions (pH 5.5-7.0) preventing the excessive microbial degradation of proteins. Tannin-protein complexes are dissociated in the acidic pH of the abomasum (pH 2.5-3.5) and in alkaline conditions of the distal small intestine (pH 7.5) and they release the protein for digestion and absorption (Barry et al., 1986).
Quebracho is the most extensively extract explored to modulate ruminal fermentation and to improve protein utilization in ruminants (Frutos et al., 2000; Hervás et al., 2003; Beauchemin et al., 2007; Benchaar et al., 2008; Aljobeili et al., 2013). Rock rose (Cistus ladanifer L.) and grape seed are plants rich in tannins and can be alternative tannin sources to improve the digestive utilisation of dietary protein. However different tannin sources can result in different nutritional responses and hence comparative studies with different tannin sources are necessary.
The objective of this study was to examine the effect of pH on the stability of complexes formed between protein and fibre from soybean meal and tannins from rock rose (Cistus ladanifer L.), grape seed (Vitis vinifera L.) and to compare with quebracho (Schinopsis quebracho-colorado Schlecht) extract.
Material and methods
Three tannin extracts were used: grape seed (La Gardonnenque SCA, France), quebracho (Unitán, Argentina) and rock rose (extract obtained in our laboratory, as described by Dentinho et al. (2007)).
Preparation of soybean meal products and washing procedure with different pH buffer solutions
Four samples of 150 g soybean meal (solvent extracted with 440 g kg-1 of crude protein (CP)) were weighed into trays. One sample was treated with 180 ml of 70% aqueous acetone (v/v) and used as control. The remaining three samples were treated using a manual sprayer with 180 ml of 70% aqueous acetone (v/v) and tannin extracts. Extract doses were 9.42, 10.95 and 15.20 g for grape seed, quebracho and rock rose, respectively, in order to obtain soybean meal products with 60 g of total phenols (TP) kg-1 of soybean meal. The amount of extract added to soybean meal was computed using TP and not CT content as it presents a significant correlation with protein precipitation capacity, higher than CT (Makkar, 2003a). Soybean meal control and soybean meal treated with grape seed extract (SG), quebracho extract (SQ) and rock rose extract (SR) were oven dried at 40 ºC for 24 h and ground to pass through a 1 mm screen. Potassium phosphate buffers (0.1 M) with pH 6 and 8 were prepared mixing appropriate amounts of solutions of potassium phosphate dibasic (1 M) and potassium phosphate monobasic (1 M) according to Green (1933). The pH 2 and pH 4 buffers were prepared from pH 6 buffer using HCl.
Control samples (4 samples) and treated soybean meal (4 samples / extract type) were weighed (4 g) for 50 ml centrifuge tubes. Thereafter, 20 ml of buffer solutions with different pH were added to centrifuge tubes and then shaken on an orbital shaker (KS 260, IKA®-Werke, Germany) for 1 h at 260 rpm and centrifuged at 1400 g for 15 min. Supernatants were collected and stored. This washing procedure was repeated three times. Residues were stored at -20 ºC and freeze dried.
Chemical analysis
The tannins extracts were analysed for TP and extractable CT. Treated and soybean meal control as well as residues after washing with buffer solutions were analysed for TP, extractable CT, protein bound CT and fibre bound CT. Total CT were calculated adding extractable CT, protein bound CT and fibre bound CT.
Extraction of phenolic compounds for TP and extractable CT determinations was carried out in four replicates, as described by Khazaal et al. (1993). Samples (200 mg) were extracted by ultrasonication (Model 200 TH/2l, VWR International, Lisboa, Portugal) at 120 W using 10 ml of 70% aqueous acetone (v/v) in an ice bath for 10 min. The obtained extract was centrifuged at 1400 g at 4ºC for 30 min and the supernatant was used for TP and extractable CT assays.
TP were analysed using Folin-Ciocalteus reagents according to Julkunen-Tiitto (1985), and concentration was measured as tannic acid equivalent using tannic acid (ref. 100 773, Merck KGaA, Darmstadt, Germany) as standard. Extractable CT were measured using the proanthocyanidin assay (butanol-HCl method) (Porter et al., 1986) and concentration was expressed as quebracho equivalents using quebracho as standard. Remaining residues from the previous extraction were dried for 12 h at 60 ºC and used for protein-bound CT and fibre bound CT determinations, according to Perez-Maldonado and Norton (1996) and the concentration was expressed as quebracho equivalents using quebracho as standard.
Soluble N in supernatants was measured according Dulphy and Demarquilly (1981).
Experimental design and statistical analysis
The experiment was a randomize complete block design with a 4 × 4 factorial treatment design, where the first factor was the type of CT extract (control – no extract, grape seed, quebracho, rock rose and the second factor was the pH of buffer solutions (2, 4, 6 and 8). The experiment was conducted in two days (block) and in each day three replicates for each treatment were performed. For TP, total CT, extractable CT, protein and fibre bound, the control treatment was excluded from the analysis, because only negligible amounts were detectable.
Data were analysed using Proc GLM of SAS (2004) according to the following model:
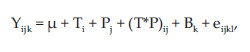
where Yij = dependent variable, µ = overall mean, T = effect of CT extract (i = 1 to 4), P = effect of pH (j = 1 to 4), (T*P)ij = interaction Ti and Pj, B = effect of day (k=1 to 2) and eijkl = residual error.
When F test was significant, multiple mean comparisons were conducted using the Tukey-Kramer test.
Results
Total phenolic contents of quebracho, grape seed and rock rose extracts were 820 g, 955 g, and 594 g of tannic acid equivalent kg-1, respectively. Table 1 shows the composition of TP, CT and nitrogen of soybean meal control and soybean meal treated with the extracts. In treated soybean meal, the recovery of TP was 63%, 38% and 26% of the expected concentration (60 g kg-1) for SQ, SG and SR, respectively.
Figure 1A shows the N solubilised by washing and recovered in supernatants for control and treated soybean meals. Figure 1B plots the differences between solubilised N from each treated soybean meal and soybean meal control, in order to emphasize the effect of tannin extracts. In control, solubilised N recovered in supernatants increased (P < 0.001) with pH, particularly for pH 8. The treatment with tannin extracts decreased (P < 0.001) solubilised N recovered in supernatants and this effect was more expressive when pH increased.
Figure 2 shows the effect of the washing buffer pH on TP and CT of tested soybean meals. The amount of TP, total CT, extractable and complexed CT (protein and fibre bound CT) was affected by the type of extract used (P < 0.001), by the buffer pH (P < 0.001) and by their interaction (P < 0.001).
Total phenolics were higher for SQ, followed by SG and finally by SR. It decreased with buffer pH but this effect was dependent of the type of extract (Figure 2A). Total CT were higher for SG, followed by SQ and for SR. As buffer pH increase the total CT decrease in SG and SR but not in SQ (Figure 2B).
For all extracts the highest values for extractable CT, expressed as g kg-1 of total CT, were obtained at pH 2 and the lowest values at pH 8. The pattern of decrease of extractable CT with pH was variable among extracts (Figure 2C). Conversely, the values for protein and fibre bound CT (as g kg-1 of total CT) were lower at pH 2 and increased with pH, and the pattern of this increase was also dependent of the type of extracts (Figure 2D and E).
In the SR most of the total CT are protein and fibre bound CT that change across pH, in a more expressive way than in SG or SQ. At pH 2 the protein and fibre bound CT were 368, 247 and 257 g kg-1 of total CT for SR, SG and SQ respectively, but at pH 8, the correspondent values were 920, 626 and 408.
With all tannins extracts the protein bound CT fraction increased from pH 2 to pH 4 and to pH 6. From pH 6 to pH 8 the protein bound condensed tannins remain unchanged in SG and SQ but increased markedly in SR.
Discussion
In soybean meals treated with tannin extracts only 63%, 38% and 26% of the total phenols (TP) added (60 g kg-1 of soybean meal) were recovered in SQ, SG and SR, respectively. The low solubility of TP in the acetone/water solution used for their extraction might explain the low recover of TP from treated soybean meals. It is well established that a variable but often important fraction of TP particularly tannins may resist to acetone/water extraction (Scalbert, 1992; Cerpa-Calderon and Kennedy, 2008; Hanlin et al., 2010). Interactions with the insoluble matrix, proteins, polysaccharides and other polymers can decrease the solubility of tannins in the extractant, underestimating tannin content of feeds. In fact, the lowest recover of TP was observed in SR, which also present the highest proportion of CT bound to protein and fibre. Nitrogen solubility was used as an indicator of soybean meal protein degradability. Although many factors, other than solubility, affect rumen degradability of proteins, nitrogen solubility is a key factor determining the protein susceptibility to microbial proteases and thus its degradability (Bach et al., 2005). Due to the good correlation observed between nitrogen degradability and solubility, this parameter has been used to predict degradability in protein rationing systems (NRC, 1985). As expected, soluble N of soybean meal was strongly pH dependent, increasing with the increase of pH, which is fully consistent with data reported by Berk (1992). According to this author, close to 80% of soybean meal protein can be extracted at neutral or alkaline conditions, and when acidity increases, solubility rapidly drops and a minimum is observed near the isoelectric region of soybean proteins (i.e. 4.2 to 4.6). In the present study, minimum N solubility for control soybean was observed at pH 4 and maintained at pH 2.
The soluble N recovered in the supernatant resulting from washing tannin treated soybean meals with different pH buffers expressed as the difference relative to control soybean meal values (Figure 1B) indicates that at pH 6 all tannins extracts clearly reduce the soluble N and that with increasing pH to 8 the quebracho is less effective in reducing the N solubility than grape seed and rock rose. These results support the possibility of using protein treated with grape seed and rock rose tannin to reduce protein degradation in the rumen (pH 5.5-7). At the lowest pH the solubility of soybean meal protein is already low and no major effects are observed with tannin treatments.
As pH increase both, protein and fibre bound CT, tended to increase and thus the extractable CT tended to decrease, although this pattern is much clearer for rock rose and less clear for quebracho. Variation in the binding capacity of different CT sources to proteins has been attributed to the different chemical structure of tannins, namely the degree of polymerization, stereochemistry and the number of phenolic hydroxyl groups (Xie and Dixon, 2005). Differences in the ability of different tannins to reduce soluble N in silages were observed by Salawu et al. (1999) and those to reduce in vitro rumen degradability of protein were observed by Aerts et al. (1999).
Makkar and Becker (1996) reported that alkaline treatments were effective to inactivate tannins in feedstuffs by diminishing the amount of extractable CT. Our data indicate that, although at mild alkaline conditions (pH 8) the recovering of extractable CT is lowest, notably for rock rose tannins, the fibre and protein tannin complex remain stable and N solubility reduction compared to control is highest. McNabb et al. (1998) reported that CT extracted from several forages (Rumex obtusifolius, Lotus corniculatus, Lotus pedunculatus, Onobrychis viciifolia, and Hedysarum coronarium) formed complexes with a soluble leaf protein (Rubisco) which also did not dissociate at pH 8.
Conclusions
The tannins of grape seed, quebracho and rock rose extracts lead to the formation of pH dependent complexes, mainly with proteins and to a lesser extent with fibre. Although the three tannin extracts presented a different binding behaviour across pH range, their ability to bind proteins in the pH range 6-8 remains consistent with the reduction of N solubility. Most of these complexes are dissociated at pH 2 and release protein. This binding pattern supports the concept of utilization of these tannin sources in ruminant nutrition as additives to protect feed protein from excessive rumen degradation. However, different efficacy among tannin sources in the protection against rumen degradation must be considered. Our results suggest that rock rose and grape seed tannins might be more effective in reducing rumen protein degradation than quebracho tannins, particularly animals fed diets promoting a higher rumen pH.
References
Aerts, R.J.; Barry, T.N. and McNabb, W.C. (1999) - Polyphenols and agriculture: beneficial effects of proanthocyanidins in forages. Agriculture, Ecosystems and Environment, vol. 75, p. 1-12. http://dx.doi.org/10.1016/S0167-8809(99)00062-6 [ Links ]
Aljobeili, H.S.; Babji, A.S. and Al-Dobaib, S.N. (2013) - Effect of various levels of quebracho tannin on the growth performance of two Saudi Arabian sheep breeds. Asia Life Science, vol. 22, n. 2, p. 459-468. [ Links ]
Bach, A.; Calsamiglia, S. and Stern, M.D. (2005) - Nitrogen metabolism in the rumen. Journal of Dairy Science, vol. 88, supplement, p. E9–E21. http://dx.doi.org/10.3168/jds.S0022-0302(05)73133-7 [ Links ]
Barry, T.N. and McNabb, W.C. (1999) - The implications of condensed tannins on the nutritive value of temperate forages fed to ruminants: a review. British Journal of Nutrition, vol. 81, n. 4, p. 263–272. [ Links ]
Barry, T.N.; Manley, T.R. and Duncan, S.J. (1986) - The role of condensed tannins in the nutritional value of Lotus pedunculatus for sheep. 4. Site of carbohydrate and protein digestion as influenced by dietary reactive tannin concentrations. British Journal of Nutrition, vol. 55, n. 1, p. 123–137. http://dx.doi.org/10.1079/BJN19860016 [ Links ]
Beauchemin, K.A.; McGinn, S.M.; Martinez, T.F. and McAllister, T.A. (2007) - Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. Journal of Animal Science, vol. 85, n. 8, p 1990–1996. http://dx.doi.org/10.2527/jas.2006-686 [ Links ]
Benchaar, C.; McAllister, T.A. and Chouinard, P.Y. (2008) - Digestion, ruminal fermentation, ciliate protozoal populations, and milk production from dairy cows fed cinnamaldehyde, quebracho condensed tannin, or Yucca schidigera saponin extract. Journal of Dairy Science, vol. 91, p. 4765–4777. http://dx.doi.org/10.3168/jds.2008-1338 [ Links ]
Berk, Z, (1992). Technology of production of edible flours and protein products from soybeans. FAO Agricultural Services, Bulletin 97. Food and Agriculture Organization of the United Nations. [cit. 2014-07-07]. www.fao.org/docrep/t0532e/t0532e07.htm [ Links ]
Bourvellec, C. and Renard, C.M.G.C. (2012) - Interactions between polyphenols and macromolecules: quantification methods and mechanisms. Critical Reviews in Food Science and Nutrition, vol. 52, n. 2. p. 213-248. http://dx.doi.org/10.1080/10408398.2010.499808 [ Links ]
Cerpa-Calderon, F.K. and Kennedy, J.A. (2008) - Berry integrity and extraction of skin and seed proanthocyanidins during red wine fermentation. Journal of Agriculture and Food Chemistry, vol. 56, n. 19, p. 9006–9014. http://dx.doi.org/10.1021/jf801384v [ Links ]
Dentinho, M.T.P; Moreira, O.C.; Pereira, M.S. and Bessa, R.J.B. (2007) - The use of a tannin crude extract from Cistus ladanifer L. to protect soya-bean protein from degradation in the rumen. Animal, vol. 1, n. 5, p. 645–650. http://dx.doi.org/10.1017/S1751731107689745 [ Links ]
Dulphy, J.P. and Demarquilly, C. (1981) - Problèmes particuliers aux ensilages In: Demarquilly C. (Ed) - Prévision de la Valeur Nutritive des Aliments des Ruminants. INRA Publications, Versailles, p. 81–104. [ Links ]
Frutos, P.; Hervás, G.; Giráldez, F.J.; Fernández, M. and Mantecón, A.R. (2000) - Digestive utilization of quebracho-treated soya bean meal in sheep. Journal of Agricultural Science, vol. 134, n. 1, p. 101–108. [ Links ]
Green, A.A. (1933) - The preparation of acetate and phosphate buffer solutions of known pH and ionic strength. Journal of the American Chemical Society, vol. 55, n. 6, p. 2331–2336. http://dx.doi.org/10.1021/ja01333a018 [ Links ]
Hagerman, A.E. (1989) - Chemistry of tannin–protein complexation. In: Hemingway, R.W. and Karchessy, J.J. (Eds.) - Chemistry and Significance of Condensed Tannins. Plenum Press; New York, p. 307–322. [ Links ]
Hanlin, R.L.; Hrmova, M.; Harbertson, J.F. and Downey, M.O. (2010) - Review: Condensed tannin and grape cell wall interactions and their impact on tannin extractability into wine. Australian Journal of Grape and Wine Research, vol. 16, n. 1, p. 173–188. http://dx.doi.org/10.1111/j.1755-0238.2009.00068.x [ Links ]
Hervás, G.; Frutos, P.; Giráldez, F.J.; Mantecón, A.R. and Álvarez, M.C. (2003) - Effect of different doses of quebracho tannins extract on rumen fermentation in ewes. Animal Feed Science and Technology, vol. 109, n. 1, p. 65-78. http://dx.doi.org/10.1016/S0377-8401(03)00208-6 [ Links ]
International Organization for Standardization (1997) - Animal feeding stuffs - determination of nitrogen content and calculation of crude protein content- Kjeldhal method, ISO 5983. [ Links ]
Julkunen-Tiitto, R. (1985) - Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. Journal of Agriculture and Food Chemistry, vol. 33, n. 2, p. 213–217. >http://dx.doi.org/10.1021/jf00062a013 [ Links ]
Khazaal, K.; Markantonatos, X.; Nastis, A. and Orskov, E.R. (1993) - Changes with maturity in fiber composition and levels of extractable polyphenols in greek browse: effects on in vitro gas-production and in sacco dry matter degradation. Journal of the Science of Food and Agriculture, vol. 63, n. 2, p. 237-244. http://dx.doi.org/10.1002/jsfa.2740630210 [ Links ]
Makkar, H.P.S. and Becker, K. (1996) - Effect of pH, temperature, and time on inactivation of tannins and possible implications in detannification studies. Journal of Agriculture and Food Chemistry, vol. 44, n. 5, p. 1291–1295. http://dx.doi.org/10.1021/jf9506287 [ Links ]
Makkar, H.P.S. (2003a) - Chemical, protein precipitation and bioassays for tannins, tannin levels and activity in unconventional feeds, and effects and fate of tannins. In: Quantification of Tannins in Tree and Shrub Foliage. A Laboratory Manual. Kluwer Academic Publishers, Dordrecht, Netherlands, p. 1-42. [ Links ]
Makkar, H.P.S. (2003b) - Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Ruminant Research, vol. 49, n. 3, p. 241–256. http://dx.doi.org/10.1016/S0921-4488(03)00142-1 [ Links ]
McNabb, W.C.; Peters, J.S.; Foo, L.Y.; Waghorn, G.C. and Jackson, F.S. (1998) - Effect of condensed tannins prepared from several forages on the in vitro precipitation of ribulose-1,5-bisphosphate carboxilase (rubisco) protein and its digestion by trypsin (EC 2.4.21.4) and chymotrypsin. Journal of the Science of Food and Agriculture, vol.77, n. 2, p. 201-212. http://dx.doi.org/10.1002/(SICI)1097-0010(199806)77 [ Links ]
Min, B.R.; Barry, T.N.; Attwood, G.T. and McNabb, W.C. (2003) - The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: a review. Animal Feed Science and Technology, vol. 106, n. 1, p. 3-19. http://dx.doi.org/10.1016/S0377-8401(03)00041-5 [ Links ]
Mueller-Harvey, I. (2006) - Unravelling the conundrum of tannins in animal nutrition and health. Journal of the Science of Food and Agriculture, vol. 86, n. 13, p. 2010–2037. http://dx.doi.org/10.1002/jsfa.2577 [ Links ]
NRC (1985) - Ruminant Nitrogen Usage. US National Academy of Science, Washington, DC, [ Links ]
Perez-Maldonado, R.A. and Norton, B.W. (1996) - The effects of condensed tannins from Desmodium intortum and Calliandra calothyrsus on protein and carbohydrate digestion in sheep and goats. British Journal of Nutrition, vol. 76, n. 4, p. 515-533. http://dx.doi.org/10.1079/BJN19960060 [ Links ]
Porter, L.J.; Hrstich, L.N. and Chan, B. (1986) - The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry, vol. 25, n. 1, p. 223-230. http://dx.doi.org/10.1016/S0031-9422(00)94533-3 [ Links ]
Porter, L.J. (1992) - Structure and chemical properties of the condensed tannins In: Hemingway, R.W. and Laks, P.E. (Eds) - Plant Polyphenols - Synthesis, Proprieties, Significance. Plenum Press, New York and London, p. 245-258. [ Links ]
Salawu, M.B.; Acamovic, T.; Stewart, C.S.; Hvelplund, T. and Weisbjerg, M.R. (1999) - The use of tannins as silage additives: effects on silage composition and mobile bag disappearance of dry matter and protein. Animal Feed Science and Technology, vol. 82, n. 3-4, p. 243-259. http://dx.doi.org/10.1016/S0377-8401(99)00105-4 [ Links ]
SAS (2004) - SAS STAT 9.1 User's Guide.SAS Institute Inc, Cary, NC. [ Links ]
Scalbert, A. (1992) - Quantitative methods for the estimation of tannins. In: Hemingway, R.W. and Laks, P.E. (Eds) - Plant Polyphenols - Synthesis, Proprieties, Significance. Plenum Press, New York and London, p. 259-280. [ Links ]
Xie, D.Y. and Dixon, R.A. (2005) - Proanthocyanidin biosynthesis- still more questions than answers? Phytochemistry, vol. 66, n. 18, p. 2126–2143. http://dx.doi.org/10.1016/j.phytochem.2005.01.008 [ Links ]
Received/recebido: 2015.05.19
Accepted/aceite: 2015.06.30














