Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias vol.37 no.4 Lisboa dez. 2014
ARTIGO
Does tree environment in agro-forestry ecosystems influence the population of N2 fixing soil bacteria?
Em ecossistemas agroflorestais terão as árvores influência nas populações de bactérias do solo fixadoras de N2?
Ricardo Soares1, Concepción Fernandéz1, Corina Carranca2, Manuel Madeira3 and Isabel Videira e Castro1
1 Laboratório de Microbiologia do Solo, Unidade Estratégica de Investigação dos Sistemas Agrários e Florestais e de Sanidade Vegetal, (UEISSAFS), INIAV,I.P., Quinta do Marquês, Av. República, Nova Oeiras, 2784-159 Oeiras, Portugal, E-mail: isabel.castro@iniav.pt, author for correspondence
2 Unidade Estratégica de Investigação dos Sistemas Agrários e Florestais e de Sanidade Vegetal (UEISSAFS), INIAV, I.P., Quinta do Marquês, Av. República, Nova Oeiras, 2784-505 Oeiras, Portugal; Biosystems Engineering (CEER), Instituto Superior de Agronomia/Universidade de Lisboa (ISA/UL), Tapada da Ajuda, Lisboa, Portugal; Mediterranean Institute for Agronomic and Environmental Studies (ICAAM), Universidade de Évora (EU), Apartado 94, 7002-554 Évora, Portugal
3 Centro de Estudos Florestais (CEF), Instituto Superior de Agronomia,Universidade de Lisboa, Tapada da Ajuda, Lisboa, Portugal
ABSTRACT
The present study aimed to monitor the rhizobial population present in four montado ecosystems from two different locations, Vaiamonte and Estremoz in the South of Portugal, with natural and sown pastures. The influence of tree canopy of Quercus suber was evaluated in relation to rhizobial population abundance, symbiotic effectiveness and genetic diversity. Results showed that the size of natural rhizobial population in the two older improved pastures analyzed, having more than 30 and 12 years, had the highest values outside the influence of cork tree canopy. However, under the canopy, the population size in these two improved pastures decreased. Also, rhizobial abundance in the youngest sown pastures (>5-years-old) had high values, either beneath the tree canopy or outside its influence. Concerning the effectiveness of symbiotic fixation, results revealed the existence of N2 fixing strains in the four pastures, the highest values being observed in the youngest improved pasture and under the tree canopy. A high genetic diversity of rhizobial population was also found in all pastures, especially outside the influence of the tree canopy.
Key-words: canopy of Quercus suber L., diversity, effectiveness, population size, rhizobia
RESUMO
O presente trabalho teve como objetivo a monitorização das populações rizobianas existentes em solos com pastagens naturais e semeadas, em quatro ecossistemas montado, de duas diferentes zonas, Vaiamonte e Estremoz, no Sul de
Portugal. Foi avaliada a influência da copa das árvores de Quercus suber, na dimensão, eficácia simbiótica e diversidade genética destas populações. Verificou-se que a influência da copa das árvores, nas duas pastagens semeadas mais antigas (há mais de 30 e de 12 anos), teve um efeito negativo na dimensão da população rizobiana do solo, com valores mais elevados fora da influência da copa dos sobreiros. A abundância da população rizobiana nas pastagens mais jovens foi também muito elevada tanto fora como debaixo da copa das árvores. Relativamente à eficácia da fixação de azoto (N), os resultados revelaram a existência de estirpes fixadoras de N nas quatro pastagens. Os valores mais elevados foram obtidos na pastagem mais jovem, semeada há mais de 5 anos, em especial debaixo da copa das árvores. Verificou-se também que a diversidade genética das populações rizobianas nas várias pastagens foi elevada, principalmente fora da influência da copa das árvores.
Keywords: Copa de Quercus suber L., dimensão da população, diversidade, eficácia, rizóbio,
Introduction
In the Mediterranean region of the Southern Iberian Peninsula a particular agro-forestry ecosystem (called montado in Portugal or dehesa in Spain) has been developed. This system is characterized, in general, by low soil fertility and by open oak formations of Quercus suber L. and Quercus rotundifolia L., being the natural and sown pastures an essential element for an extensive livestock production associated with the exploitation of cork and holm oaks. A model for improving pastures in the montado ecosystem started to be developed in Portugal in the late 1960s and was largely adopted since then, spreading all over the world throughout similar Mediterranean environments. The strategy is based on the establishment of biodiverse permanent pasture including selected and improved plant species, in which inoculated legumes with high efficiency rhizobia are the dominant plant species (Castro and Ferreira, 2011; Castro et al., 2007; Ferreira and Castro, 2001). These biodiverse legume-rich mixtures are well adapted for semi-arid areas and provide a better pasture productivity than the natural flora and are able to renew themselves on a permanent basis (Crespo, 2006).
Biological nitrogen fixation (BNF) mediated by symbioses between soil bacteria, collectively known as rhizobia, and plant legumes is a major component in the improvement of agricultural sustain-ability, having pasture legumes an important role (Materon, 1988).
In the montado ecosystems the BNF is the main process of supplying N to the soil and is therefore a key component of a strategy for increase the productivity of these ecosystems (Ferreira et al., 2010). Nodulation and N2 fixation in this symbiosis require that host and microorganisms are compatible, but also that the soil environment is appropriated for the exchange of signals that precede infection (Hirsch et al., 2003; Zhang et al., 2002). Also, in the Rhizobium-legume symbiosis, the process of N2 fixation is strongly related to the physiological status of the host plant. In addition, environmental factors may affect the genetic and phenotypic characteristics of rhizobial populations present in the soil and can, in some cases, reduce rhizobial survival and diversity in soil or even affect the growth of the host plants. For example, low (<8 ºC) and high (>35 ºC) temperatures may be considered one of major causes of nodulation failure, affecting all stages of the symbiosis and limiting rhizobial growth and survival in soil (Carranca, 2013; Hungria and Franco, 1993).
Therefore, a competitive and persistent Rhizobium strain is not expected to express its full capacity for N2 fixation if limiting factors (e.g. inadequate photosynthesis, salinity, unfavorable soil pH, nutrients (e.g. P, K, Mo, B) deficiency, mineral (heavy metals) toxicity and contaminants, temperature extremes, insufficient or excessive soil moisture, plant diseases, and intensive grazing impose limitations to the host (Brockwell, 1981; Brockwell et al., 1995; Carranca, 2013; Peoples et al., 1995). It is known that tree canopy influences both the quality and quantity of light reaching the understory vegetation in a limited area of these systems, which has the effect of decreasing the density of the pasture sward (Wilson and Ludlow, 1991) and can also influence the composition of underlying soil microbial communities (Grayston et al., 2001). The identification of the structural and functional features of microbial communities, in particular of N2 fixing bacteria, inhabiting soils of agro-forestry ecosystems with different pasture composition and age, can be helpful in order to define the impact of the tree environment on the activity of these soil microorganisms (Marongiu et al., 2006). The present study aimed to monitor the rhizobial population present in soils of four different montado ecosystems: one with a natural p 12asture and three with improved pastures. These systems were chosen to evaluate the influence of the cork oak (Q. suber) tree canopy in several parameters of soil rhizobial community, including the abundance, symbiotic effectiveness and genetic diversity, using subterranean clover (Trifolium subterraneum L.) as host plant, which is one of the legume species more commonly used in the installation of upland pastures and suitable annual legume with self re-seeding capacity for soil conservation in these ecosystems.
Materials and Methods
Sites description and layout of the experiment
The study was developed in four montado ecosystems located at South of Portugal: a natural pasture (>25 years old) and two improved pastures (respectively 5 and 12 years old), at Estremoz, and an improved pasture (>30 years old), at Vaiamonte. In these sites the climate is characterized by dry hot summers and wet cool winters. The mean annual rainfall was about 800 mm at Vaiamonte and about 600 mm at Estremoz. Soils had a loamy-sand texture and were classified as District Cambisols and Eutric Luvisols overlying granitic bedrock (IUSS, 2006), respectively at Vaiamonte and Estremoz. Soils showed a slightly acid reaction (pH(H2O)=5.5) which did not vary among pastures but increased outside the tree canopy (pH(H2O)=5.8). Total N and carbon (C) also did not differ significantly among pastures (1.3 and 17 g kg-1, respectively for total N and C), but were significantly higher beneath the tree canopy (1.8 and 23 g kg-1, respectively for total N and C) (data not shown).
Improved pastures were sown in the autumn with different species of Trifolium L. such as T. subterraneum, T. vesiculosum Savi, T. incarnatum L., T. resupinatum L., T. michelanium Savi, and also with Ornithopus sativus Brot., Lolium multiforum Lam. and Dactylis glomerata L., at a rate of 25-30 kg seeds per hectare. Legume seeds were previously inoculated with Rhizobium strains (provided by Fertiprado®). In November 2010, several plots (1.2 m2) were arranged in annual rotational grazing pastures in the aforementioned montado ecosystems. The layout of the experiment was a split-plot design with three replicates. Two sets of plots were randomly installed in each site. One set consisted of three plots randomly distributed under the tree canopy and another set consisted of three randomized plots distributed outside the tree influence. To restrict the access of cattle (cows, sheep, pigs) for grazing the herbage, experimental sites were protected with fences and cages during the study period.
Soil sampling
Soils samples were collected aseptically in December 2010, at 0-20 cm depth in each plot and stored in sealed plastic bags under cooled conditions. Afterward, soil samples were thoroughly mixed, passed through a 2 mm sieve to remove stones and large pieces of organic matter and stored in the refrigerator (6 oC) till microbiological analyses were processed.
Rhizobial abundance
The size of rhizobial population was estimated by indirect count, using the most-probable-number (MPN) method and a ten-fold dilution series. T. subterraneum cv. Clare growing in Jensen's agar medium was used as host test plant (Vincent, 1970). Plants were grown for 8 weeks in a controlled environmental room (18-20 oC, 12 hours light/day). After this period, plants were harvested and roots were examined for nodulation. The results were expressed as log10 of rhizobia bacteria number per gram of dried soil (Somasegraran and Hoben, 1994).
Symbiotic effectiveness
The "whole-soil inoculation technique" (Brockwell et al., 1988; Quigley et al., 1997) was used to assess the nitrogen fixing potential of the soil rhizobial population. Results obtained from shoot dry matter production (measured after oven drying at 70 oC) of subterranean clover nodulated plants, previously inoculated with 10-1 and 10-2 dilutions of each soil sample, allowed to differentiate the rhizobial resident populations according to their symbiotic effectiveness.
The values of shoots dry weight (X) were used to calculate the index of effectiveness (E) following the criterion used by several authors (Beck et al., 1983; Ferreira and Marques, 1992) as follows: Ej (%) = (XjXT0/XTN-XT0)x100, where j is the inoculated soil dilution, TN the nitrogen control (plants received 0.05% KNO3) and T0 the uninoculated control (without soil and without mineral nitrogen). Three levels of effectiveness were adopted by these authors: ineffective (E<25%), effective (E=25% and <74%) and highly effective (E=75%).
Genetic diversity
ERIC (Enterobacterial repetitive intergeneric consensus)-PCR technique (De Brujin, 1992; Versalovic et al., 1994) was used to study the genetic diversity of the rhizobial population. This technique is used to distinguish strains taxonomically very close and has been successfully applied in studies of Rhizobium sp. diversity (Lorite et al., 2012; Castro and Ferreira, 2006). About 80 isolates of Rhizobium leguminosarum biovar trifolii obtained from nodules of subclover plants grown under laboratorial conditions were used in the PCR reactions, after extraction of total DNA, and the respective fingerprintings were visualized by horizontal electrophoresis on agarose gels. Results were analyzed using the binary system related to the presence/absence of fragments. Matrices of the Dice coefficient were calculated, and cluster analysis was performed using the UPGMA (Unweighted Pair Group with Arithmetic Average) algorithm and the program Free Tree (Efron et al., 1996; Pavlicek et al., 1999). The program Tree View (PHILIP) was used for the construction of dendrograms and evaluation of the respective genetic relationships.
Genetic diversity was evaluated by calculating the Simpsons index (D) (Simpson, 1949), which varies between 0, when all isolates belong to the same genomic group, and 1, when each isolate corresponds to a different genomic group.
Results
Rhizobial population size
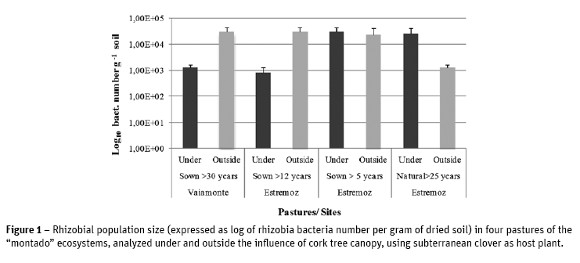
The results of rhizobial population abundance showed a decline in this population in the plots that were under the influence of cork oaks canopy, with mean values of 1.3 x 103 and 8.16 x102 bacteria g-1 soil, respectively in Vaiamonte (> 30-years-old) and Estremoz (> 12-years-old), compared with plots outside the influence of tree canopy, where an average value of 3.06 x 104 bacteria g-1 soil in both pastures was quantified. In the other Estremoz pastures (sown for more than 5 years, and natural stand older than 25 years / under the influence of the canopy of oaks) the size of rhizobial population was always high and superior to 104 bacteria g-1 soil, except for the natural pasture outside the tree influence with low value (Figure 1).
Nitrogen fixing capacity
Results obtained from dry weight of sub cloverplants inoculated with soil samples dilutions collected in the four pastures were always higher than the control T0 (uninoculated plants), but lower than the control with mineral nitrogen (TN) (Figure 2).
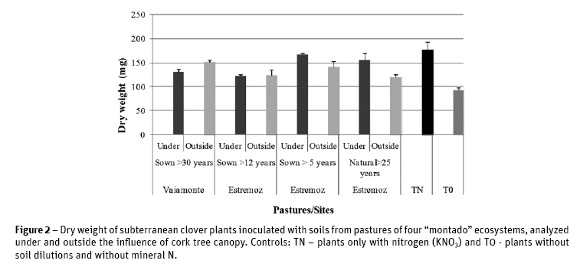
These results indicated the existence of N2 fixing bacteria in the four pastures, although rhizobial population in each pasture had different nitrogen
fixing capacity.
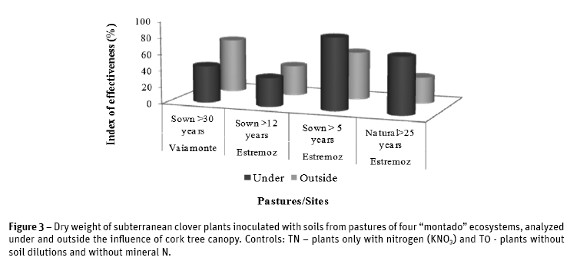
Values of shoots dry weight were used to calculate the index of effectiveness. The results showed the inexistence of ineffective (E<25%) rhizobial population in all the pastures (Figure 3). Results also showed that soil rhizobial population from younger pasture sown for more than 5 years had the highest index of effectiveness (E= 88%), under the tree canopy, and was the unique pasture having a highly effective population (E =75%). Inversely, the lowest index of effectiveness (E=32%) was obtained in the natural pasture for the rhizobial population outside the tree canopy.
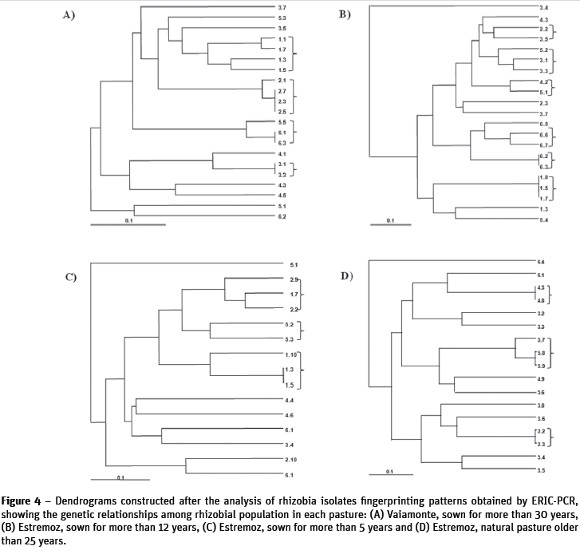
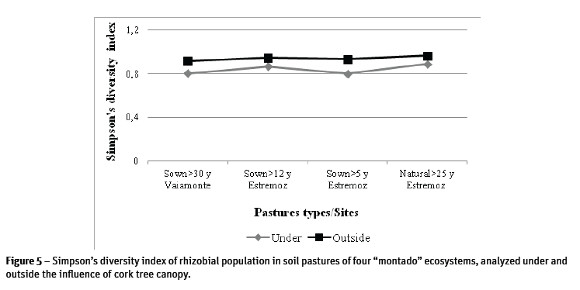
Results of genetic diversity of rhizobial population in the different pastures, represented in the form of dendrograms (Figure 4) showed the existence of multiple clusters in the studied population. Many of the Rhizobium isolates produced a single and complex fingerprinting demonstrating the existence of a great diversity within the population. The Simpsons diversity index (D) of ERIC-PCR profiles of rhizobial population in each pasture was determined at 85% minimum similarity (Figure 5). Presented results confirmed the existence of high diversity especially in rhizobia population outside the influence of the tree canopy and from sown pastures where D=0.9 was close to the maximum limit (D=1.0). Under the influence of cork oak canopy, the values of diversity index in improved pastures were lower (between 0.8 and 0.7). In natural pastures, values under and outside of the influence of tree canopy were similar and high (Figure 5).
Discussion
Soil microorganisms are critical to the maintenance of soil functions, in both natural and managed agricultural ecosystems. This explains the importance of evaluating a particular group of microorganisms, such as legume root nodule bacteria. In this one-year study, the influence of cork oak trees in rhizobial communities associated with clovers present in different pastures was investigated. Rhizobial population size was one of the studied parameters more negatively affected, particularly in the older and sown pastures under the effect of the tree canopy. The importance of rhizobial population in soils in which legumes are either present or have been recently cultivated has been reported by several researchers. The size of these populations has been reported to vary between < 10 and 107 bacteria g-1 soil, although rhizobial populations fluctuates through the year with higher numbers in spring and lower numbers in autumn (sowing time), after a dry summer (Bottomley, 1992; Ferreira et al., 2010; Pryor and Crush, 2006; Vincent, 1974). Results obtained in our study showed a decrease in the abundance of rhizobial populations in the older and sown pastures, under the influence of tree canopy, when compared with the results obtained outside the influence of tree canopy. In the remaining pastures the results indicated a rhizobial population size greater than 104 bacteria g-1 soil, in the autumn, which could be considered enough for an efficient nodulation.
These results are in agreement with those reported by Ferreira et al. (2010) which showed that the size of rhizobial population in similar ecosystems was around 5 x 104 bacteria g-1 soil in the autumn.
Besides the abundance of the nodulating rhizobial populations, their symbiotic effectiveness with hosts is fundamental in satisfying the N requirements of the legume plants, particularly when soil N is depleted. However, an ample range of symbiotic effectiveness can be found and several authors have shown that soil rhizobial populations can present a great variability for N2 fixation (Gibson et al., 1975). Furthermore, ineffective symbioses are considered a natural phenomenon, in most agro-ecosystems, due to legume infection by ineffective Rhizobium strains (Vincent, 1981). For example, Ferreira and Marques (1992) estimated that 15% of rhizobia isolates of subterranean clover, collected from several Portuguese soils were ineffective or poorly effective in N2 fixation. In the present study the symbiotic efficiency in all pastures had higher values than the uninoculated controls, indicating the presence of N2 fixing Rhizobium strains. As a general trend, the age and type of pastures (sown and natural) as well as the influence of the cork oak canopy did not affect the ability of N2 fixation by rhizobial population, although the highest values were obtained in the youngest pasture (sown for more than 5 years). This pasture was the unique having a highly effective population (E =75%), probably due to relatively recent inoculation of subterranean clover seeds with R. leguminosarum bv. trifolii. Inversely, the minor index of effectiveness (E=32%) was obtained for rhizobial population in the natural pasture.
In a recent study, the role of BNF on a large number of long term natural pastures in Portuguese montado ecosystems covering different edaphoclimatic conditions was investigated (Ferreira and Castro, 2011). Although the amount of N2 fixed was highly variable among sites, the results showed that N2 fixation was closely linked to the legume biomass production. This study also confirmed that the legume productivity in natural pastures was very low, as a result of poor natural flora. Present results showed that, in general, symbioses were well adapted to these environmental conditions and the introduction of improved pastures legumes, previously inoculated with selected rhizobia strains represents an effective way of increasing pastures productivity, particularly noticed in the youngest pastures.
Various tools have been used to explore the genotypic variation among the rhizobial population and to assess their diversity (Castro et al., 1997; Durán et al., 2013; Laguerre et al., 1994; Lorite et al., 2012; Thies, 2007). In the present study, ERIC-PCR was used to assess whether the influence of tree canopy lead to changes in rhizobia diversity. Results indicated the existence of multiple molecular fingerprinting patterns among rhizobia isolates, demonstrating that there was no selection for a single genotype, even in younger improved pasture inoculated with Rhizobium, where no single rhizobiagenotype appeared to have established dominance over the other genotypes. These results can be explained by the existence of horizontal gene transfer among rhizobial population, which is generally recognized as an important factor in rhizobial diversity and occurs frequently among native and introduced population (Wernegreen et al., 1997). This is also one of the important reasons why changes in the genetic structure of soil populations of rhizobia can occur within a decade (Graham, 2009). These data reveal a high genetic diversity found in the rhizobial population isolated from subterranean clover from all pastures, although genetic diversity was, in general, smaller under the cork oak canopy and are in agreement with those reported by other authors (Garbeva et al., 2004).
Results of the present study indicate that tree environment might have a negative impact especially in the oldest improved pastures, which may not be as productive under the influence of the tree canopy due to the degree of shade suffered over several years after its installation. This trend is in agreement with that reported by several authors (Ouma and Jeruto, 2010; Shelton et al., 1987; Sogbedji et al., 2006), who pointed out the level of shade as one the most significant factor determining the pastures output, suffering legumes more from shading than grasses and being also affected N2 fixation process. Therefore the decision to install improved pastures in montado areas may take into account the degree of canopy cover.
Conclusions
Plant species are a major determinant of the structure of microbial communities in soil, as plants are the main providers of specific carbon and energy sources and in the particular case of legume plants, they influence the composition of underlying soil microbial communities, mainly the rhizobial population. Cork oak canopy also influences directly the development of pastures and so exerting its effects on rhizobial population. The four agro-forestry montado ecosystems under study, including pastures with different ages and composition (improved and natural) showed the influence of cork oak tree in N2 fixing bacterial population, mainly in the size, which was negatively affected, particularly in older and sown pastures. Also, soil rhizobial population diversity was, in general, smaller under the effect of the cork oak canopy. These N2 fixing soil bacteria were particularly efficient in the symbiosis with the youngest sown pasture legumes, whereas the lowest symbiotic efficiency was observed in the natural pasture. Nowadays, legumes are considered important components of the strategy for increasing production and sustainability of the montado ecosystem and symbiotic N2 fixation is a major process of providing N to the soils.
Thus, factors affecting this process should be take in account and avoided.
Acknowledgements
The research leading to these results has received funding from the Portuguese Foundation for Science (FCT) under Grant Agreement STRAW-PTDC/AGR-AAM/102369/2008.
References
Beck, D.P.; Materon, L.A. and Afandi, F. (1993) - Practical Rhizobium legume technology manual, Technical Manual No: 19. Aleppo, Syria, International Center for Agricultural Research in the Dry Areas (ICARDA), 54 p. [ Links ]
Bottomley, P.J. (1992) -Ecology of Rhizobium and Bradyrhizobium. In: Stacey, G.; Burris, R.H. and Evans, H.J. (Eds.) - Biological Nitrogen Fixation. New York, Chapman & Hall, p. 293-348. [ Links ]
Brockwell, J. (1981) - A strategy for legume nodulation research in developing regions of the world. Plant and Soil, vol. 58, n. 1-3, p. 367-382. [ Links ]
Brockwell, J.; Bottomley, P.J. and Thies, J.E. (1995) - Manipulation of rhizobia microflora for improving legume productivity and soil fertility: a critical assessment. Plant and Soil, vol. 174, n. 1-2, p. 143-180. [ Links ]
Brockwell, J.; Holliday, A.R. and Pilka, A. (1988) -Evaluation of the symbiotic nitrogen-fixing potential of soils by direct microbiological means. Plant and Soil, vol. 108, n. 1, p. 163-170. [ Links ]
Carranca, C. (2013) - The role of legumes for sustainability: Research needs for future prospects. In: Camisão, A.H. and Pedroso, C.C. (Eds.) - Symbiosis: Evolution, Biology and Ecological Effects. New York, Nova Science Publishers, p. 177-188. [ Links ]
Castro, I.V. and Ferreira, E.M. (2006) -Contaminación y fertilización: metales pesados y lodos de depuradoras. In: Gómez, E.B.; Lóperz, J.G.; Plá, C.L. and González, M.B.R. (Eds.) - Fijación de Nitrógeno: Fundamentos y Aplicaciones. Granada, Spain. Sociedad Española de Fijación de Nitrógeno (SEFIN), p. 291-303. [ Links ]
Castro, I.V. and Ferreira, E.M. (2011). Inoculantes microbianos para leguminosas. In: Coelho, P.S. and Reis, P. (Eds.) - Agrorrural. Contributos Científicos. Portugal, Instituto Nacional dos Recursos Biológicos, I.P. e Imprensa Nacional – Casa da Moeda, S.A., p. 298-307. [ Links ]
Castro, I.V.; Ferreira, E.; Machado, H.; Duarte, I. and Tavares de Sousa, M. (2007) - O uso de biofertilizantes em Portugal. In: Izaguirre-Mayoral, M.L.; Labandera, C. and Sanjuan, J. (Eds,) -Biofertilizantes em Iberoamérica: una visión técnica, cientifica y empresarial. Montevideo, Uruguay, BIOFAG/ CyTED, p. 68-74. [ Links ]
Castro, I.V.; Ferreira, E.M and McGrath, S.P. (1997) - Effectiveness and genetic diversity of Rhizobium leguminosarum bv. trifolii isolates in portuguese soils polluted by industrial effluents. Soil Biology and Biochemistry, vol. 29, n. 8, p. 1209-1213. [ Links ]
Crespo, D.G. (2006) - The role of pasture improvement on the rehabilitation of the montado/dehesa system and in developing its traditional products. In: Ramalho Ribeiro, J.M.C.; Horta, A.E.M.; Mosconi, C. and Rosati, A. (Eds.) - Animal Products from the Mediterranean area. EAAP publication Nº 119. Wageningen, The Netherlands Academic Publishers, p. 185-197. [ Links ]
Durán, D.; Reya, L.; Sánchez-Cañizaresa, C.; Navarro, A.; Imperial, J. and Ruiz-Argueso, T. (2013) - Genetic diversity of indigenous rhizobial symbionts of the Lupinus mariae-josephae endemism from alkaline-limed soils within its area of distribution in Eastern Spain. Systematic and Applied Microbiology, vol. 36, n. 2, p. 128-136. [ Links ]
De Bruijn, F.J. (1992) - Use of repetitive (Repetitive Extragenic Palindromic and Enterobacterial Repetitive Intergenic Consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Applied and Environmental Microbiology, vol. 58, n. 7, p. 2180-2187. [ Links ]
Efron B.; Halloran, E. and Holmes, S. (1996) - Bootstrap confidence levels for phylogenetic trees. Proceedings of National Academic Science US, vol. 93, p. 13429-13434. [ Links ]
Ferreira, E.M. and Castro, I.V. (2001) -Production and application of legume inoculants in Portugal: Peat-based inoculants for clover seeds. In: Koch, E. and Leinonem, P. (Eds.) -Formulation of Microbial inoculants. Brussels, European Commission, Cost Action 830 - EUR 19692, p. 94-97. [ Links ]
Ferreira, E.M. and Castro, I.V. (2011) -Fijación bi-ológica de nitrógeno y productividade de pastos naturales en sistemas agrícolas del mediterráneo. In: Megías, M; Rivilla, R.; Soto, M.J.; Delgado, M.J.; González, E.; Mateos, P.F.; León, M.; Rodelas, B. and Bedmar, E.J. (Eds.) - Fundamentos y Aplicaciones agroambientales de las interacciones beneficiosas plantas-microorganismos. Spain, Sociedad Española de Fijación de Nitrógeno (SEFIN), p. 403-416. [ Links ]
Ferreira, E. and Marques, J.F. (1992) - Selection of Portuguese Rhizobium leguminosarum bv. trifolii strains for production of legume inoculants. I. Screening for effectiveness in laboratory conditions. Plant and Soil, vol. 147, n. 1, p. 151-158. [ Links ]
Ferreira, E.M.; Simões, N.; Castro, I.V. and Carneiro, L.C. (2010) - Relationships of selected soil parameters and natural pastures yield in the Montado ecosystem of the Mediterranean area using multivariate analysis. Silva Lusitana, vol. 18, n. 2, p. 151-166. [ Links ]
Garbeva, P.; Van Veen, J.A. and Van Elsas, J.D. (2004) -Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annual Review of Phytophatology, vol. 42, p. 243-270. [ Links ]
Gibson, A.H.; Curnow, B.C.; Bergerssen, F.J.; Brock-well, J. and Robinson, A.C. (1975) -Studies of field populations of Rhizobium: Effectiveness of strains of Rhizobium trifolii associated with Trifolium subterraneum L. in south-eastern Australia. Soil Biology and Biochemistry, vol. 7, n. 2, p. 95-102. [ Links ]
Grayston, S.J.; Griffith, G.S.; Mawdsley, J.L.; Campbell, C.D. and Bardgett, R.D. (2001) - Accounting for variability in soil microbial communities of temperate upland grassland ecosystems. Soil Biology and Biochemistry, vol. 33, n. 4-5, p. 533 - 551. [ Links ]
Graham, P.H. (2009) -Soil biology with emphasis on symbiotic nitrogen fixation. In: Emerich, D.W. and Krishnan, H.B. (Eds.) -Nitrogen Fixation in crop production. Madison, USA, American Society of Agronomy, p. 171-209. [ Links ]
Hirsch, A.M.; Bauer, W.D.; Bird, D,M,; Cullimore, J.; Tyler, B. and Yoder, J.J. (2003) - Molecular signals and receptors: controlling rhizosphere interactions between plants and others organisms. Ecology, vol. 84, n. 4, p. 858-868. [ Links ]
Hungria, M. and Franco, A.A. (1993) -Effects of high temperatures on nodulation and N2 fixation in Phaseolus vulgaris, L.. Plant and Soil, vol. 149, n. 1, p. 95-102. [ Links ]
IUSS Working Group (2006) - World reference base for soil resources 2006, Vol.103, World soil resources report, 2ª Edição, Roma, FAO. [ Links ]
Laguerre, G.; Allard, F. R. and Amarger, N. (1994) -Rapid Identification of rhizobia by restriction fragment length polymorphism analysis of PCR-amplified 16S rRNA Genes. Applied and Environmental Microbiology, vol. 60, n. 1, p. 56-63. [ Links ]
Lorite, J.; Castro, I.V.; Muñoz, S. and Sanjuán, J. (2012) - Phylogenetic relationship of Lotus uliginosus symbionts with bradyrhizobia nodulating genistoid legumes. FEMS Microbiology Ecology, vol. 79, n. 2, p. 454-464. [ Links ]
Marongiu, R.; Garau, G.; Caredda, M. and Deiana, P. (2006) -Impact of Soil Management on the Functional Activity of Microbial Communities associated to Cork Oak Rhizosphere. IEEE, p. 46-50. [ Links ]
Materon, L.A. (1988) - Maximizing Biological Nitrogen Fixation by Forage and Pasture Legumes in Semi-Arid Areas. In: Beck, D.P. and Materon, L.A. (Eds.) -Nitrogen Fixation by legumes in Mediterranean agriculture. Syria, ICARDA, Developments in Plant and Soil Sciences, cap.32, p. 33-40. [ Links ]
Ouma, G. and Jeruto, P. (2010) - Sustainable horticultural crop production through intercropping: The case of fruits and vegetable crops: A review. Agriculture and Biology Journal of North America, vol. 1, p. 1098-1105. [ Links ]
Pavlicek, A.; Hrda, S. and Flegr, J. (1999) - Free-Treefreeware program for construction of phylogenetic trees on the basis of distance data and bootstrap/jackknife analysis of the tree robustness. Application in the RAPD analysis of genus Frenkelia. Folia Biologica, vol. 45, n. 3, p. 97 - 99. [ Links ]
Peoples, M. B.; Ladha, J. K. and Herridge. D. F. (1995) -Enhancing legume N2 fixation through plant and soil management. Plant and Soil, vol. 174, n. 1-2, p. 83–101.
Pyror, H.N. and Crush, J.R. (2006) - Elevated populations of effective rhizobia in the rhizoplane of white clover growing in pasture. New Zealand Journal of Agriculture Research, vol. 49, n. 1, p. 85-87. [ Links ] Quigley, P.E.; Cunningham, P.J.; Hannah, M.; Ward, G.N. and Morgan, T. (1997) - Symbiotic effectiveness of Rhizobium leguminosarum bv. trifolii collected from pastures in south-western Victoria. Australian Journal of Experimental Agriculture, vol. 37, n. 6, p. 623-630. [ Links ]
Shelton, H.M.; Humphreys, L.R. and Batello, C. (1987). Pastures in the plantations of Asia and the Pacific: performance and prospect. Trop. Grassl., vol. 21, n. 4, p. 159-168. [ Links ]
Simpson, A. (1949) - Measurement of diversity. Nature, vol. 163, p. 688. [ Links ]
Sogbedji, J.M.; Van, H.M.; Melkonian, J.R.R. and Schindelbeck, R.R. (2006) -Evaluation of the P. N. M. model for simulating drain flow nitrate-N concentration under manure fertilized maize. Plant and Soil, vol. 282, n. 1-2, p. 343-360. [ Links ]
Somasegaran, P. and Hoben, H.J. (1994) - Handbook for Rhizobia: Methods in legume-Rhizobium techonology. New York, Springer-Verlag, 450 p. [ Links ]
Thies, J.E. (2007) - Molecular methods for studying soil ecology. In: Paul, E.A. (Ed.) – Soil Microbiology, ecology and biochemistry. 3rd ed. Amsterdam, Academic Press, p. 85-118. [ Links ]
Vincent, J.M. (1970) – A Manual for the practical study of the root-nodule-bacteria. IBP Handbook No. 15. Oxford, Blackwell Scientific Publications, 164 p. [ Links ]
Vincent, J.M. (1974) -Root-nodule symbioses with Rhizobium. In: Quispel, A. (Ed.). The Biology of nitrogen fixation. Amsterdam, North-Holland Pub. Co., p. 265-341. [ Links ]
Vincent, J.M. (1981) - The genus Rhizobium. In: Starr, M.P.; Stolp, H.; Truper, G.H.; Balows, A. and Schlegel, H.G. (Eds.) - The Prokaryotes. A handbook on habitats, isolation and identification of Bacteria. Berlin, Germany, Springer-Verlag, vol. 1, p. 818-841. [ Links ]
Versalovic, J.; Schneider, M.; De Bruijn, F.J. and Lupski, J.R. (1994) - Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods in Cell Biology, vol. 5, n. 1, p. 25-40. [ Links ]
Wernegreen, J.J.; Harding, E.E. and Riley, M.A. (1997) - Rhizobium gone native: unexpected plasmid stability of indigenous Rhizobium leguminosarum. Proceedings of the National Academy of Sciences USA, vol. 94, n. 10, p. 5483–5488. [ Links ]
Wilson, J.R. and Ludlow, M.M. (1991) -The environment and potential growth of herbage under plantations. In: Shelton, H.M. and Stur, W.W. (Eds.) - Forage for Plantation crops. Canberra, ACIAR Proceedings, cap.32, p.10-24. [ Links ]
Zhang, H.H.; Charles, T.C.; Driscoll, B.T.; Prithiviraj, B. and Smith, D.L. (2002) - Low temperature-tolerant Bradyrhizobium japonicum strains allowing improved soyabean yield in short-season areas. Agronomy Journal, vol. 94, n. 4, p. 870-875. [ Links ]
Recebido/Received: 2014.05.20
Aceite/Accepted: 2014.10.06














