Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de Ciências Agrárias
Print version ISSN 0871-018X
Rev. de Ciências Agrárias vol.36 no.4 Lisboa Oct. 2013
ARTIGO
Effect of substrates and plant growth promoting bacteria in the germination of sugarcane seeds
Efeito de substratos e bactérias promotoras do crescimento vegetal na germinação de sementes de cana-de-açúcar
Guilherme Grodzki O. Figueiredo1, Valéria Rosa Lopes2, João Carlos B. Filho2 e Edelclaiton Daros2
1 Department of Plant Science and Crop Protection, Federal University of Paraná, Rua dos Funcionários, 1540 – Curitiba, Paraná, Brazil, Email: ggofigueiredo@outlook.com; guigrodzki@yahoo.fr, author for correspondence
2 Department of Plant Science and Crop Protection, Federal University of Paraná, Brazil
ABSTRACT
The aim of this work was to test different substrates with Plant Growth Promoting Bacteria (PGPB) inoculation on sugarcane seed germination. The substrates were sand, vermiculite and Plantmax®. The completely randomized factorial design 2x3, with 4 repetitions was used. The parameters estimated were speed of germination index, days for emergence, and 30 days after sowing the parameters: height of seedlings (cm), volume of roots (cm3), length of roots (cm), and the number of germinated plants. The application of PGPB promoted better development of seedlings, mainly roots. The Plantmax® presented the better conditions for germination and seedling development. Vermiculite had the worst results. No response to PGPB was observed in the sand. The use of Plantmax® and PGPB in germination of sugarcane seeds is recommended.
Keywords: PGPB, vigor, Saccharum spp., seedling, Plantmax®
RESUMO
O objetivo do presente trabalho foi testar diferentes substratos e a inoculação com bactérias promotoras do crescimento vegetal (BPCV) na germinação de sementes de cana-de-açúcar. Os susbtratos foram areia, vermiculita e Plantmax®. O delineamento foi inteiramente casualizado em fatorial 2x3, com 4 repetições. Foram estimados os parâmetros índice de velocidade de germinação, dias para emergência, e aos 30 dias após a semeadura os parâmetros: altura das plântulas (cm), volume das raízes (cm3), comprimento das raízes (cm) e o número de plântulas germinadas. A aplicação de BPCV promoveu o crescimento das plântulas, principalmente das raízes. O Plantmax® apresentou as melhores condições para o desenvolvimento das plântulas e para a germinação. Na vermiculita o desenvolvimento das plântulas foi limitado. Na areia não houve resposta à aplicação de BPCV. Recomenda-se a utilização do substrato Plantmax® e a aplicação de BPCV na germinação de sementes de cana-de-açúcar.
Palavras-chave: BPCV, vigor, Saccharum spp., plântula, Plantmax®
Introduction
The Sugarcane (Saccharum spp.) is one of the main crops planted in the world. Its commercial planting is through vegetative propagation, and the boundaries of its cultivation are close to the Palm Tree Line (James, 2004). The Sugarcane is widely used in the sugar and ethanol production (Matsuoka et al., 2005). For the development of the culture, the breeding programs look for cultivars better adapted to the environments, with higher yields and diseases resistance (Landell and Bressiani, 2008). This process may last 13 years (Barbosa and Silveira, 2010).
The sugarcane breeding has many distinct steps, and one of the first steps is controlled crosses. The crosses are made at Sugarcane Flowering and Crossing Stations and true seeds (caryopsis) are produced (Cabral, 2007). The true or viable seed (caryopsis) is shed within the spikelet, inside of lemma and palea. If the seed is non-viable, the spikelet does not have caryopsis inside. When many seeds are together, they are known as fuzz (Cabral, 2007; James, 2004). The seeds are sent to Experimental Stations in the Breeding Programs, where will be sown according the local methodology.
Many papers about sugarcane caryopsis were produced after the 60s (Chilton et al., 1965; Herbert et al., 1962; Silva, 1977). Cabral et al. (2011) and Caieiro et al. (2010) studied the viability and germination of crosses. Cabral (2007), observed the increase in vigor and germination percentage in fuzz of sugarcane with application of gibberellic acid (GA3). Other papers with sugarcane seeds were published; however most studies were focused mainly in contamination for fungus (Cazalet and Berjak, 1983; Martins, et al., 2009; Sanguino and Tokeski, 1980), storage (Cabral et al., 2011; Caieiro, 2008; Rao, 1982) and seed processing (Bleicher and Tokeshi, 1980; Corte Brilho and Tokeshi, 1992).
Few works have studied substrates and environments in sugarcane seed germination. Kwon-Ndung and Imolehin (2007) evaluated substrates in germination of sugarcane caryopsis and Silva et al. (2010) tested different environments (laboratory and greenhouse) in sowing seeds. Many works focusing substrates and germination are generally in forest seeds (Gasparin et al., 2012; Martins et al., 2012), fruits (Negreiros et al., 2005; Nogueira et al., 2013) and currently in seeds with high oil levels (Pascuali et al., 2012; Santos et al., 2013).
The use of nitrogen fertilizers in sugarcane cultivars is important to the nutrition and promotion of productivity and development of culture (Vitti et al., 2008). However, many studies showed that the incorrect handling of these fertilizers may pollute watercourses and atmosphere (Beaulieu et al., 2010; Howden et al., 2013; Liu et al., 2011). One alternative to substitute the nitrogen fertilizers, totally or partially, might be the use of inoculants of diazotrophic bacteria or Plant Growth Promoting Bacteria (PGPB) (Bashan and Holguin, 1998). These bacteria may fix nitrogen by mechanism of biological nitrogen fixation (BNF) (Boddey et al., 2001; Urquiaga et al., 1992), and/or promote the vegetal growth of plants, through production of growth regulators, phosphate solubilization and other mechanisms (Beneduzi et al., 2013; Lira-Cadete et al., 2012; Taulé et al., 2012). In sugarcane seeds, Madhaiyan et al. (2005) tested methylobacterial strains and verified the increase of true seed germination.
Urquiaga et al. (2012) estimated that cultivars of sugarcane obtain at least 40 kg N ha-1 yr-1 from association with diazotrophic bacteria. Many experiments demonstrated these bacteria are able to growth promotion and biological nitrogen fixation (BNF) in sugarcane (Silva et al., 2009; Silva et al., 2012; Pereira et al., 2013; Schultz et al., 2012). Notwithstanding these experiments, the studies with sugarcane and PGPB are often with commercial cultivars (Pereira et al., 2013; Schultz et al., 2012).
Some studies have focused on genotype-bacterium interaction in sugarcane, through BNF response from genotypes with different bacteria/strains (Caballero-Mellado and Munõz-Rojas, 2003). For this reason, breeding aiming BNF could be one way to increase its efficiency (Lopes et al., 2012), although it is necessary previous studies with sugarcane seeds and its viability for diazotrophic bacteria inoculation and the interactions with substrate.
These factors justify works in sense to utilize PGPB in caryopsis sowing, aiming better quality of seeds and helping breeding in future works.
The objective of this work was to test different substrates with PGPB inoculation in seeds of sugarcane.
Materials and methods
The work was performed in growth room with 25 °C ± 2 of temperature, and 16 hours of photoperiod. It was utilized the sugarcane cross (family) 472B (RB931003 x RB001913), year 2009. The cross came from the Sugarcane Flowering and Crossing Station in Serra do Ouro of Ridesa (Interuniversity Network for the Development of Sugarcane Industry), Alagoas State, Brazil.
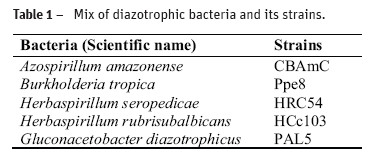
For sowing, it was used fuzz and the plot was represented by one pot with 1000 cm3 capacity, with 650 cm3 of substrate. There was 150 mg of fuzz per plot. Three sterile substrates were used: two non-commercials (sand and vermiculite) and the commercial substrate Plantmax®. The label of the product shows that the commercial substrate is consisted of vermiculite, pinus bark, simple superphosphate and potassium nitrate. According to Negreiros et al. (2005), the chemical characteristics of the Plantmax® are: pH (H2O) 5.47, 662.1 ppm P, 600 ppm K, 22.62 ppm Zn, 210.3 ppm Fe, 21.4 ppm Mn, 0.79 ppm Cu, 9.64 (cmolc dm-3) Ca2+, 3.65 (cmolc dm-3) Mg2+ and 0.24 (cmolc dm-3) Al3+. The substrates were autoclaved at 1 atm, 120 °C for 60 minutes, to eliminate any microorganism that may interact with inoculant, as recommended by Brasil (2009) to the sand substrate. One sieve with 710 µm was utilized to standardize the sand. The treatments were control (non-inoculated) and inoculated with peat inoculant of diazotrophic bacteria (Table 1), of which were mixed 5 g of inoculants and 650 cm3 of substrate. The inoculant had 109 bacteria g-1.
To evaluate the treatments and substrates the following parameters were estimated:
Days for emergence (DE) (Edmond and Drapala, 1958) were calculated by number of days to germinate first seed.
Speed of germination index (SGI) (Maguire, 1962), using the formula:

Number of germinated seedlings was calculated by sum of number of germinated seeds in the plots 30 days after sowing (DAS).
Height of seedlings (cm) was determined by size of seedlings, measured with ruler 30 days after sowing DAS.
Total plot length (cm) and volume (cm3) of roots – The roots were analyzed using the computer program Win/MacRhizo version 4.1c.
The completely randomized factorial design 2 x 3 (two treatments and three substrates) was used, with four replications. It was applied F-test and the treatments means were compared by Tukey test (P<0.05). Data was analyzed using the statistical software SISVAR® version 5.0, from Federal University of Lavras, Brazil (Ferreira, 2011).
Results and discussion
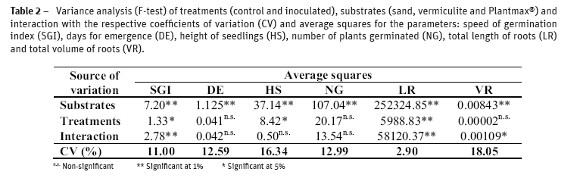
The analysis of variance showed significant difference to substrates in all parameters. For treatments, significant differences were detected only for speed of germination index (SGI), height of seedlings and length of roots. Significant interaction was detected for the parameters SGI, total length and volume of roots (Table 2). Altogether, the inoculant treatment was better than control, although inoculation showed negative interaction in vermiculite substrate for some parameters (Fig. 1A;B;C).
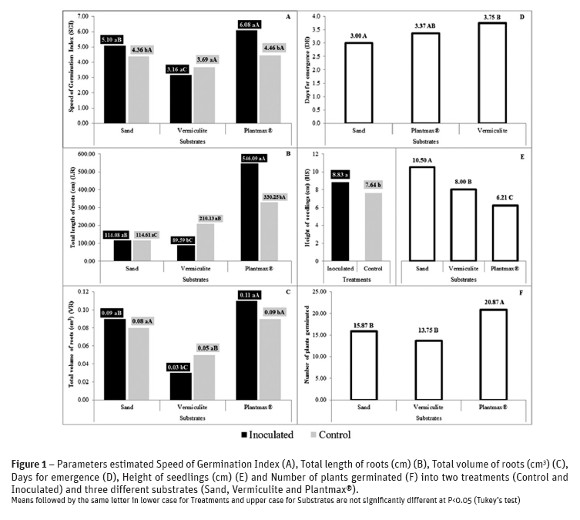
The Fig. 1A shows that for SGI in the control (not inoculated plants), there were no differences between substrates, while the application of inoculants resulted in better response in Plantmax® followed by sand. Plantmax® and sand showed positive interaction with inoculants treatment, unlike vermiculite that there was no response for the inoculants application. Studying germination of seed cane (vegetative propagation with stalks), Silva et al. (2012) observed better sprouting of buds when applying mixed bacteria in the sugarcane cultivar RB72454. Madhaiyan et al. (2005) verified the increase of sugarcane caryopsis germination with inoculation of methylobacterial strains. The authors also demonstrated in leaves of clone Co86032 higher cytokinin contents.
The behavior of substrates was different for days for emergence (DE). The sand provided the conditions for seeds to germinate first. Plantmax® had the intermediate value and vermiculite was the last one (Fig. 1D). Silva et al. (2010) also worked with SGI and DE evaluating sugarcane caryopsis germination and reported significant differences in both variables to different conditions of temperature.
It was observed better development in the aerial part of seedling (factor substrate) in sand substrate, followed respectively by Vermiculite and Plantmax® (Fig. 1E). Opposite to this result, in the commercial substrate a greater number of plants germinated after 30 days, followed respectively by vermiculite and sand (Fig. 1F). This result may be related the competition of plants in the plots, with Plantmax® having more competition than the others substrates. In the sand, where the seedlings were taller, less competition may have occurred due to the low number of plants germinated.
Utilizing substrates Plantmax® and sand, Martins et al. (2012) did not find difference on height of seedlings of Schizolobium parahyba. Until 14 DAS the seedlings in the commercial substrate were highest than sand and pine sawdust. According to the work of Negreiros et al. (2005), the Plantmax® presented minor values of height of seedlings in papaya tree when compared with other substrates.
Plantmax® was the best substrate for length of roots comparing other substrates in both treatments, the sand exhibited lower length of roots in control treatment, and when inoculated sand presented the intermediate value. Vermiculite was intermediate in control treatment and the last one when inoculated (Fig. 1B). To the factor treatment, no difference was found on sand, the best treatment in the vermiculite was control and in the Plantmax® the inoculated treatment. The volume of roots (Fig. 1C) has followed the same tendency, excepted the substrate vermiculite that obtained the minor values to the factor substrates. Vermiculite interacted negatively and Plantmax® interacted positively in both parameters with the inoculant (Fig. 1B,C).
Probably, the higher amount of nutrients and better physical conditions in Plantmax® may influence the results of this work. Negreiros et al. (2005) working with papaya tree seedlings, observed the greater length of roots with the same substrate. Smirdele et al. (2001) have demonstrated the good conditions to germination of the Plantamax® substrate, where the accumulation of roots dry mass and seedlings were better than mixed substrates, in lettuce, cucumber and red pepper. Diniz et al. (2006) also verified benefits in tomato, pepper and lettuce seedlings. Catunda et al. (2008) have concluded that the physical characteristics of the commercial substrate, as higher retention of water, low density and higher aeration increases the averages of dry mass of the roots.
Know-Ndung and Imolehin (2007) verified better response of vermiculite in the germination of sugarcane caryopsis, however the cost of the substrate may be expensive. In this way, they justified the mixture of different substrates and vermiculite.
The presence of PGPB in inoculated treatments may produce growth regulators that implying the better development of roots and may be help on the growth of aerial part of plant as on the present work (Fig. 1E). One of those growth regulators produced by bacteria is the auxin (Taulé et al., 2011), acting in the growth of roots, enlarging the absorption area, and increasing the contribution of nutrients presents in soils (Taiz and Zeiger, 2010). That effect may be related with better length and volume roots of the inoculated commercial substrate (Fig. 1B,C). Working with lettuce, Schlindwein et al. (2008) verified better germination and roots length of the inoculate treatments with bacteria that produce IAA.
The authors Bashan and Holguin (1997) also reported benefits of PGPB inoculation on root growth and attributed through production of IAA (indol acetic acid) of Azospirillum genus. The A. amazonense strain CBAmC, one of the bacteria that compound the bacteria mix (Table 1), demonstrated its potential to IAA production by Reis Junior et al. (2004), this growth regulator may be associated to the better development of roots in inoculated treatment. On the other hand Taulé et al. (2011) did not find IAA production to the same bacteria, but they have found IAA production in Gluconacetobacter diazotrophicus and Herbaspirillum seropedicae,and phosphate solubilization in Burkholderia tropica, all of them are also part of the bacteria mix.
Conclusions
The application of the inoculant based on PGPB demonstrated its potential to apply in sugarcane caryopsis. Among the substrates, the Plantmax® demonstrated better condition to development of caryopsis and great interaction. Generally the growth of seeds in sand has been good, however it did not presented conditions to development of caryopsis combined with bacteria. The vermiculite was not good for the development of seeds in both treatments. According the results, Plantmax® is recommended and vermiculite is not recommended to use in studies with sugarcane seeds.
Acknowledgements
The author Guilherme Figueiredo thanks REUNI for a scholarship. The authors want to thanks INCT – Fixação Biológica do Nitrogênio for financial support, Embrapa Agrobiologia (Seropédica-RJ) for supplying inoculants; to researchers from Ridesa: Prof. Geraldo Veríssimo de Souza Barbosa and Biologist MSc. Luiz José Oliveira Tavares de Melo for providing sugarcane seeds; and Prof. Dr. Ricardo Augusto de Oliveira and Prof. Dr. José Luis Camargo Zambon for the help on the analysis of the data.
References
Barbosa, M.H.P. and Silveira, L.C.I. (2010) – Melhoramento genético e recomendação de cultivares. In: Santos, F.; Borém, A. and Caldas, C. – Cana-de-açúcar: bioenergia, açúcar e álcool – tecnologia e perspectivas. Viçosa, UFV, p. 313-331. [ Links ]
Bashan, Y. and Holguin, G. (1998) - Proposal for the division of plant growth-promoting rhizobacteria into two classifications: bio-control-PGPB (plant growth-promoting bacteria) and PGPB. Soil Biology & Biochemistry, vol.30 p. 1225–1228. [ Links ]
Beaulieu, J.; Tank, J.L.; Hamilton, S.K.; Wollheim, W.M.; Hall Jr., R.O.; Mulholland, P.J.; and Thomas, S.M. (2010) - Nitrous oxide emission from denitri?cation in streamand river networks. PNAS (Online). (Accessed at July 22, 2013). Available at http://www.pnas.org/cgi/doi/10.1073/pnas.1011464108. [ Links ]
Beneduzi, A.; Moreira, F.; Costa, P.B.; Vargas, L.K.; Lisboa, B.B.; Favreto, R. and Passaglia, L.M.P. (2013) - Diversity and plant growth promoting evaluation abilities of bacteria isolate from sugarcane cultivated in the South of Brazil. Applied Soil Ecology, vol. 63, n. 1, p. 94-104. [ Links ]
Bleicher, J. and Tokeshi, H. (1980) - Effect of ventilation on germination and vigor of sugarcane caryopses. In: Proceedings of the XVII International Society of Sugar Cane Technologists Congress. Manila, Philippines, ISSCT, p. 1250-1255. [ Links ]
Boddey, R.M.; Polidoro, J.C.; Resende, A.S.; Alves, B.J.R. and Urquiaga, S. (2001) - Use of the 15N natural abundance technique for the quantification of the contribution of N2 fixation to sugar cane and other grasses. Australian Journal of Plant Physiology, vol. 28, n. 9, p. 889–895. [ Links ]
Brasil, Ministério da Agricultura, Pecuária e Abastecimento (2009) - Regras para Análise de Sementes. Secretaria de Defesa Agropecuária. Mapa/ACS [ Links ]
Cabral, F.F. (2007) - Qualidade fisiológica e armazenamento de sementes de cana-de-açúcar provenientes de diferentes cruzamentos. Master dissertation. Rio Largo, Universidade Federal de Alagoas, 62p. [ Links ]
Cabral, F.F.; Silva, C.B.; Ferreira, V.M.; Araújo Neto, J. C. and Barbosa, G.V.S. (2011) – Fertilidade de cruzamentos, potencial fisiológico e armazenamento de sementes de cana-de-açúcar. Revista Brasileira de Tecnologia Aplicada nas Ciências Agrárias, vol. 33, n. 1, p. 66-82. [ Links ]
Caieiro, J.T. (2008) – Avaliação da qualidade de sementes (cariopses) de cana-de-açúcar (Saccharum spp.) como suporte ao melhoramento genético. Master dissertation. Curitiba, Universidade Federal do Paraná, 55p. [ Links ]
Caieiro, J.T.; Panobianco, M.; Bespalhok Filho, J.C. and Ohlson, O.C. (2010) - Physical purity and germination of sugarcane seeds (caryopses) (Saccharum spp.). Revista Brasileira de Sementes, vol. 32, n. 2, p. 140-145. [ Links ]
Catunda, P.H.A.; Marinho, C.S.; Gomes, M.M.A. and Carvalho, A.J.C. (2008) - Brassinoesteróides e substratos na aclimatização do abacaxizeiro imperial. Acta Scientiarum Agronomy, vol 40, n. 3, p. 345-352. [ Links ]
Cazalet, K.R. and Berjak, P. (1983) – Isolation of a seed storage fungus from sugarcane seeds. In: Proceedings of the South African Sugar Technologists Association. SASTA, p. 105-108. [ Links ]
Chilton, J.P.; Paliatseas, E.D. and Perdomo, R. (1965) – Production of true seed of sugarcane in Louisiana. In: Proceedings of the XII International Society of Sugar Cane Technologists Congress. San Juan, Puerto Rico, ISSCT, p. 785-789. [ Links ]
Corte Brilho, F.F. and Tokeshi, H. (1992) - Assessment of the cleaning of sugarcane caryopses. In: Proceedings of the XXI International Society of Sugar Cane Technologists Congress. Bangkok, Thailand, ISSCT, p. 468-475. [ Links ]
Diniz, K.A.; Guimarães, S.T.M.R. and Luz, J.M.Q. (2006) – Húmus como substrato para a produção de mudas de tomate, pimentão e alface. Bioscience Journal, vol. 22, n. 3, p. 63-70. [ Links ]
Edmond, J.B. and Drapala, W.J. (1958) - The effects of temperature, sand and soil, and acetone on germination of okra seeds. In: Proceedings of the American Society Horticultural Science. Alexandria, United States of America, ASHS, p. 428-443. [ Links ]
Ferreira, D. F. (2011) – Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia (UFLA), vol. 35, n. 6, p. 1039-1042. [ Links ]
Gasparin, E.; Araujo, M.M.; Avila, A.L. and Wielewicki, A.P. (2012) - Identificação de substrato adequado para germinação de sementes de Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. Ciência Florestal, vol. 22, n. 3, p. 625-630. [ Links ]
Herbert, L.P; Breaux, R.D. and Fanguy, H.P. (1962) – Bunch-planting experiments with sugar cane seedlings at the U.S. sugar cane field station, Houma, LA. In: Proceedings of the XI International Society of Sugar Cane Technologists Congress. Reduit, Mauritius, ISSCT, p. 553-560. [ Links ]
Howden, N.J.K.; Burt, T.P.; Worrall, F.; Mathias, S.A. and Whelan, M.J. (2013) – Farming for water quality: Balancing food security and nitrate pollution in UK river basins. Annals of the Association of American Geographers, vol.103, n.2 p.397-407. [ Links ]
James, G. (2004) – An Introduction to Sugarcane. In: James, G. (Ed.) – Sugarcane. Oxford, Blackwell Science Ltd, p. 1-19. [ Links ]
Kwon-Ndung, E.H. and Imolehin, E.D. (2007) – Evaluation of sugarcane seedlings from biparental crosses using different growth substrates. In: Proceedings of the African Crop Science Society Conference. El-Minia, Egypt, ACSS, p. 139-142. [ Links ]
Landell, M.G.A. and Bressiani, J.A. (2008) – Melhoramento genético, caracterização e manejo varietal. In: Dinardo-Miranda, L.L.; Vasconcelos, A.C.M. and Landell, M.G.A. – Cana-de-Açúcar. Campinas, Instituto Agronômico, p. 101-155. [ Links ]
Lira-Cadete, L.; Farias, A.R.B.; Ramos, A.P.S.; Costa, D.P.; Freire, F.J. and Kuklinsky-Sobral, J. (2012) - Variabilidade genética de bactérias diazotróficas associadas a plantas de cana-de-açúcar capazes de solubilizar fosfato inorgânico. Bioscience Journal, vol. 28, n. 1, p. 122-129. [ Links ]
Liu, X.; Duan, L.; Mo, J.; Du, E.; Shen, J.; Lu, X.; Zhang, Y.; Zhou, X.; He, C. and Zhang, F. (2011) - Nitrogen deposition and its ecological impact in China: An overview. Enviromental Pollution, vol. 159, n. 10, p. 2251-2264. [ Links ]
Lopes, V.R.; Bespalhok Filho, J.C.; Araujo, L.M.; Rodrigues, F.V.; Daros, E. and Oliveira, R.A.(2012) - The selection of sugarcane families that display better associations with plant growth promoting rhizobacteria. Journal of Agronomy, vol. 11, n. 2, p. 43-52. [ Links ]
Madhaiyan, M.; Poonguzhali, S.; Lee, H.S.; Hari, K.; Sundaram, S.P. and Sa, T.M. (2005) - Pink-pigmented facultative methylotrophic bacteria accelerate germination, growth and yield of sugarcane clone Co86032 (Saccharum officinarum L.). Biology and Fertility of Soils, vol. 41, n. 5, p. 350-358. [ Links ]
Martins, C.C.; Borges, A.S.; Pereira, M.R.R. and Lopes, M.T.G. (2012) - Posição da semente na semeadura e tipo de substrato sobre a emergência e crescimento de plântulas de Schizolobium parahyba (vell.) s.f. blake. Ciência Rural, vol. 22, n. 4, p.845-852. [ Links ]
Martins, T.D.; Menten, J.O.M. and Sanguino, A. (2009) - Fungos associados às sementes (Cariopses) de cana-de-açúcar: métodos para detecção, incidência e relação entre incidência fúngica e ambiente de produção das sementes. Summa Phytopathologica, vol. 35, n. 3, p. 173-178. [ Links ]
Matsuoka, S.; Garcia, A.A.F. and Arizono, H. (2005) – Melhoramento da Cana-de-Açúcar. In: Borém, A. (Ed.) – Melhoramento de espécies cultivadas. Viçosa, UFV, p. 225-274. [ Links ]
Munõz-Rojas, J. and Caballero-Mellasdo, J. (2003) – Population dynamics of Gluconacetobacter diazotrophicus in sugarcane cultivars and its effect on plant growth. Microbial Ecology, vol. 46, n. 4, p. 454-464. [ Links ]
Negreiros, J.R.S.; Braga, L.R.; Álvares, V.S. and Bruckner, C.H. (2005) - Diferentes substratos na formação de mudas de mamoeiro do grupo solo. Revista Brasileira Agrociência, vol. 11, n. 1, p. 101-103. [ Links ]
Nogueira, N.W.; Ribeiro, M.C.C.; Freitas, R.M.O.; Gurgel, G.B. and Nascimento, I.L. (2013) - Diferentes temperaturas e substratos para germinação de sementes de Mimosa caesalpiniifolia Benth. Revista de Ciências Agrárias, vol. 56, n. 2, p.95-98. [ Links ]
Pascuali, L.C.; Silva, F.S.; Porto, A.G.; Silva Filho, A. and Meneghello, G.E. (2012) - Germinação de sementes de pinhão manso em diferentes temperaturas, luz e substratos. Semina: Ciências Agrárias, vol. 33, n. 4, p.1435-1440. [ Links ]
Pereira, W.; Leite, J.M; Hipólito, G.S.; dos Santos, C.L.R. and Reis, V.M. (2013) – Acúmulo de biomassa em variedades de cana-de-açúcar inoculadas com diferentes estirpes de bactérias diazotróficas. Revista Ciência Agronômica, vol. 44, n. 2, p. 363-370. [ Links ]
Rao, P.S. (1982) - Sugarcane seed storage for breeding and generic conservation. In: Seminário Inter Americano de la caña de azúcar – VARIEDADES. Flórida International University. Miami, USA. [ Links ]
Sanguino, A. and Tokeshi, H. (1980) – Fungus pathology in sugarcane caryopses. In: Proceedings of the XVII International Society of Sugar Cane Technologists Congress. Manila, Philippines, ISSCT, p. 1555-1562. [ Links ]
Santos, V.M.; Castro, H.G.; Cardoso, D.P.; Leal, T.C.A.B. and Lima, S.O. (2013) - Avaliação de tipos de substratos no crescimento inicial de variedades de mamoneira. Journal of Biotechnology and Biodiversity, vol. 4, n. 1, p. 60-69. [ Links ]
Schultz, N.; Morais, R.F.; Silva, J.A.; Baptista, R.B.; Oliveira, R.P.; Leite, J.M. and Reis, V.M. (2012) - Avaliação agronômica de variedades de cana-de-açúcar inoculadas com bactérias diazotróficas e adubadas com nitrogênio. Pesquisa Agropecuária Brasileira, vol. 47, n. 2, p. 261-268. [ Links ]
Silva, M.A.; Caputo, M.M.; Perecin, D. and Bressiani, J.A. (2010) – Comparação de ambientes na germinação de cariopses de cana-de-açúcar. Ciência e Agrotecnologia, 34, Edição Especial: 1604-1609. [ Links ]
Silva, M.F.; António, C.S.; Oliveira, P.J.; Xavier, G.R.; Rumjanek, N.G.; Soares, L.H.B. and Reis, V.M. (2012) – Survival of endophytic bacteria in polymer-based inoculants and efficiency of their application to sugarcane. Plant and Soil, vol. 356, n. 1-2, p. 231-243. [ Links ]
Silva, M.F.; Oliveira, P.J.; Xavier, G.R.; Rumjanek, N.G. and Reis, V.M. (2009) – Inoculantes formulados com polímeros e bactérias endofíticas para a cultura da cana-de-açúcar. Pesquisa Agropecuária Brasileira, vol. 44, n. 11, p. 1437-1443. [ Links ]
Silva, W.M. (1977) - Production of sugarcane seedlings by the method of fuzz processing and early transplantation. In: Proceedings of the XVI International Society of Sugar Cane Technologists Congress. São Paulo, Brazil, ISSCT, p. 165-176. [ Links ]
Smiderle, O.J.; Salibe, A.B.; Hayashi, A.H.; Minami, K. (2001) - Produção de mudas de alface, pepino e pimentão em substratos combinando areia, solo e plantmax. Horticultura Brasileira, vol. 19, n. 3, p. 253-257. [ Links ]
Taulé, C.; Mareque, C.; Barlocco, C.; Hackembruch, F.; Reis, V.M.; Sicardi, M. and Battistoni, F. (2012) - The contribution of nitrogen fixation to sugarcane (Saccharum officinarum L.), and the identification and characterization of part of the associated diazotrophic bacterial community. Plant and Soil, vol. 356, n. 1-2, p.35-49. [ Links ]
Urquiaga, S.; Cruz, K.H.S. and Boddey, R.M. (1992) - Contribution of nitrogen fixation to sugarcane: nitrogen-15 and nitrogen balance estimates. Soil Science Society of America Journal, vol. 56, n.1, p. 105-114. [ Links ]
Urquiaga, S.; Xavier, R.P.; Morais, R.F.; Batista, R.B.; Schultz, N.; Leite, J.M. and Boddey, R.M. (2012) - Evidence from field nitrogen balance and 15N natural abundance data for the contribution of biological N2 fixation to Brazilian sugarcane varieties. Plant and Soil, vol. 356, n. 1-2, p. 5-21. [ Links ]
Vitti, A.C.; Cantarella, H.; Trivelin, P.C.O. and Rossetto, R. (2008) – Nitrogênio. In: Dinardo-Miranda, L.L.; Vasconcelos, A.C.M. and Landell, M.G.A. – Cana-de-Açúcar. Campinas, Instituto Agronômico, p. 239-269. [ Links ]
Recebido/Received: 2013.08.09
Aceitação/Accepted: 2013.09.17














