Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias v.33 n.2 Lisboa dez. 2010
Nitrogen nutrition of young triticale plants grown under aluminium stress
Ana Maria Domingues1, 2
1Tropical Research Institute, Department of Natural Sciences / Instituto de Investigação Científica Tropical, Departamento de Ciências Naturais Tapada da Ajuda – PAIAT, 3 rd floor/ 3º piso Apartado 3014, 1301-901 Lisboa Codex PORTUGAL
2Centro de Estudos da Macaronésia / Center for Macaronesian Studies, University of Madeira, Campus Penteada, 9000-390 Funchal - PORTUGAL E-mail: dam.portugal@hotmail.com
ABSTRACT
Triticale has proven to be a tolerant crop in many places around the globe, under extreme climatic and edaphic conditions, particularly in Al-toxic soils. To suffice the growing food demand of the world population, one of the most important goal is the sustainable increase of cereal production avoiding the anthropogenic pollution, often air and groundwater contamination by volatilization and leaching of N compounds.
The effects of enhanced ammonia proportion in relation to nitrate nutrition in hydroponics, with or without Al, were investigated on the short-term growth of triticale plants. Three days old plants of the Al-tolerant genotype TTE 9203 were submitted to 0 or 370 mM Al and received different NO3-/ NH4+ ratios with the four proportions 15:1, 8:1, 3:1 and 1:1 (with fixed total N concentration at 3.2 mM in all treatments).
In relation to the corresponding control solutions, 370 mM Al induced important decreases in root length, ranging from 75.3 % to 47.3 %, reductions in fresh weight from 80 % to 60 % in roots, and from 89 % to 71 % in shoots, depending on the NO3-/ NH4+ ratio. A decrease in NO3- net uptake was shown by plants in the presence of Al. The most detrimental Al effect for young plant growth in nutrient solutions was observed with the 15:1 NO3-/ NH4+ ratio, which induced the highest reductions of length of the main root (52.7 % reduction relative to control) and of root biomass fresh weight (40.6 %) in four days of treatment. By the contrary, the plants grown in the 8:1 ratio solution with Al suffered the smallest reductions of root length (24.7 % in 370 mM Al treatment relative to control) and of root biomass fresh weight (20.3 %). Taken together the results indicate that NH4+ can alleviate Al toxicity in triticale and point out the ideal NO3-/ NH4+ proportion of 8:1 as the best for these young plants growth and N use efficiency, under acidic and Al toxic condition.
Some economical and ecological advantages of NH4+- N sources use in plant fertilization are discussed.
Keywords: Aluminium tolerance, ammonium, nitrate, triticale.
Adubação azotada de plântulas de triticale sob toxicidade de alumínio
RESUMO
O triticale constitui uma cultura tolerante a condições climáticas e edáficas extremas, estando bem adaptado a solos ácidos, com níveis tóxicos de Al. A satisfação das necessidades cerealíferas crescentes da população mundial é o desafio fundamental da agricultura sustentável, evitando a poluição antropogénica, frequentemente por compostos azotados contaminantes de aquíferos e da atmosfera.
Foi estudado o enriquecimento amoniacal na adubação azotada em triticale, na presença de alumínio tóxico. As plantas com três dias do genótipo tolerante ao Al TTE 9203 foram sujeitas à presença de 0 ou 370 mM Al e receberam as quatro proporções de NO3-/ NH4+ seguintes 15:1, 8:1, 3:1 e 1:1, tendo sido fixada a concentração total de 3,2 mM N nas diferentes modalidades em solução nutritiva.
Relativamente aos correspondentes controlos, os tratamentos com 370 mM Al induziram reduções importantes no crescimento, que variaram significativamente entre 75,3% e 47,3% do comprimento radicular, 80% a 60% da biomassa radicular e 89% a 71% da biomassa aérea, segundo a proporção de NO3-/ NH4+ presente na solução nutritiva. Efectivamente, o Al tóxico reduziu muito significativamente a absorção de nitrato pelas plantas. O efeito negativo do Al nas jovens plantas foi mais evidente na modalidade com a proporção 15:1 NO3-/ NH4+, tendo a presença de Al provocado as reduções de 52,7 % do alongamento da radícula mais longa e de 40,6 % da biomassa fresca de raiz produzida em quatro dias de tratamento. Pelo contrário, na modalidade com a proporção 8:1 NO3-/ NH4+, as raízes das plantas sofreram as reduções mínimas daqueles parâmetros relativamente ao correspondente controlo (24,7 % e 20,3 %, respectivamente). No seu conjunto, os resultados obtidos indicaram que o NH4+ pode aliviar a toxicidade do Al em triticale, na fase vegetativa precoce que constitui a mais susceptível à toxicidade daquele metal. Sob stress por Al, a proporção de NO3-/ NH4+ ideal para o crescimento das plântulas e maior eficiência de uso do azoto será aproximadamente de 8:1.
São discutidos aspectos económicos e ecológicos da utilização de fontes alternativas de azoto amoniacal na fertilização da cultura.
Palavras-chave: Amónia, nitrato, tolerância ao alumínio, triticale.
INTRODUCTION
Adapted plant genotypes may give economic returns and are specially interesting in developing countries, under stress or sub-optimal conditions (Bozzini, 1991; Ceccarelli, 1996). Triticale (xTriticosecale Wittm.), which is an amphydiploid of the crossing between wheat and rye, often includes cultivars characterised by resistance to diseases, exhibiting good performance under marginal climatic and edaphic conditions, particularly high grain yield in poor acid soils showing high saturation with Al toxic ions (Aniol, 1996). It constitutes a nutritious staple food for human consumption (Bozzini, 1991). The sustainable increase of agricultural production is the primordial goal to suffice the growing food demand of the world population (Yusuf et al., 2003). This includes to increase the efficient use of nutrients by plants and also to reduce the environmental pollution associated, which might be achieved in tropical agro forestry systems (Preto, 1983).
N is available on Earth mainly as the chemically stable diatomic-N (N2). But there are many reactive N species which concentrations are increasing in the environment (Fields, 2004), as the N oxides (N2O and NOx), volatile ammonium (NH3), ionic-N forms (NO3- , NO2- and NH4+) and biological-N forms (Jones & Willett, 2006). However, in conventional agriculture, undoubtedly mineral NO3- and NH4+ are the most important N-forms for non-Leguminosae plant nutrition (Glass et al., 2002). Nitrogen is the most limiting nutrient for plant production, but the incorrect fertilizers applications induce atmospheric and groundwater contamination by this macronutrient. Nitrogen losses may occur by leaching and surface runoff, by volatilisation, and by biological and chemical transformations. Soil N losses are proportional to the excess of supply relative to plants needs (Shaviv, 2000). The nitrate and the toxic nitrite N-forms are most susceptible to leaching and runoff (Silgram & Shepherd, 1999). It must be emphasized that fertiliser production, transport, distribution, and, specially in what concerns NO3--N, the mineral nutrient availability to fill up plant stages needs along the cultural cycle are expensive. The N-fertilizers effective uptake by roots and N allocation in the plant is progressive in time, so often the profits of a single or few NO3--N applications are exiguous and the effects on environment are negative, especially in many tropical region soils (Rosenzweig & Hillel, 2000).
The optimal NO3-/ NH4+ ratio applied in N-fertilisation depends on many factors, as the total N-availability (in both mineral and organic forms), plant species preferences, edaphic and climatic conditions (specially water regime and temperature), and agronomic management. Under acidic conditions, enhanced ammonia (NH4+) nutrition favoured bigger earnings of wheat (Fleming, 1983), N assimilation in the seeds of barley (Soares & Lewis, 1986) and yield of maize (Alexander et al., 1991). The Spring barley yields were similar with applications of NH4NO3 or (NH4)2SO4 (with proportions 1:1 or 0:1 of NO3-/ NH4+ ratio) in acidic soils, with and without liming (Malhi et al., 1988). However, in nutrient solutions, these authors found that the barley plants produced more biomass when they were supplied with only NO3--N compared to only NH4+–N. In another hydroponic experiment, Vaast et al. (1998) found that at 20º C and with 1 mM NH4+ (as sole mineral N sources), the N uptake of coffee, Coffea arabica L. cv. Catuai Vermelho, doubled between pH 2.75 and 7.25. The NO3--N uptake was more reduced than that of NH4+-N at temperatures below 16º C and in anaerobic conditions.
Acid conditions shift the chemical stability of the ubiquitous Al compounds in soils and dramatically rise the concentration of soluble forms of Al. Blamey et al. (1983) considered that the activities of AlOH2+, Al(OH)30, and AlSO4+ were low in solution and suggested that generally the Al3+ and Al(OH)2+ ions might be the predominant species responsible for decreased root elongation. The Al concentration as low as 37 to 74 mM in soil solutions can damage the plant roots and disturb the metabolic systems, e.g., provoke impaired cation nutrients uptake (Roy et al., 1988). Moreover, monomeric and polymeric Al forms might induce very detrimental rhizotoxic effects (Blamey et al., 1983; Andrade et al., 1996).
After 3-4 days of treatment with 74 mM Al in nutrient solution, initially at pH 4.5 and containing 4 mM N at 12.4:1 of NO3-/ NH4+ ratio, the differential Al susceptibility of wheat cultivars (Triticum aestivum L.) was evident by visual symptoms in the roots (Taylor & Foy, 1985). Al tolerance negatively correlated with the high rate of NH4+ depletion and it was positively correlated with the moderate rate of NO3- depletion and, consequent, gradual rise of the pH root media. The authors concluded that cultivars preference for NO3--N, and the corresponding raise in the pH of the solution, significantly contributed to Al tolerance of those genotypes. Nevertheless, varieties of bread wheat grown hydroponically with mixed N-forms (1mM NO3- plus 0.3 mM NH4+) accumulated less Al in the roots compared to those grown only with 1.3 mM NO3--N (Andrade et al., 1996).
The nitrate reductase and nitrite reductase activities of roots and shoots of pearl millet (Pennisetum typhoids L. ou Pennisetum glaucum (L.) R. Br.) seedlings declined, as well the NO3--N, soluble protein and chlorophyl contents in tissues, in Al-treated plants (Albassam, 2001). The presence of Al in the root media reduced uptake and reduction of nitrate, particularly in the more susceptible cultivar of rice (Justino et al., 2006).
The objectives of this study were to investigate the effects of different proportions of NO3-/ NH4+ in nutrient solutions with 3.2 mM N, added with 0 or 370 mM Al, on the early growth and N net uptake of triticale. The nutrient solution method used in a short-term experiment, under strictly controlled conditions, pretends to contribute to a better adjustment of the nitrogen fertilisation needs of Al-tolerant triticale germplasm grown under very acidic and Al-toxic conditions in sustainable agriculture.
MATERIALS AND METHODS
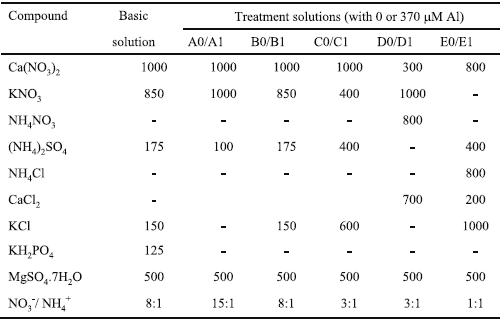
Seeds of the genetic line of triticale (xTriticosecale Wittm.; amphydiploid hybrid from Family Poacea) TTE 9203 from Estação Nacional de Melhoramento de Plantas (Elvas), were surface sterilised with 5% sodium hypochlorite for eight minutes, washed with distilled water and were germinated over water imbibed cotton covered with filter paper, at 20 ± 3º C. Seedlings having 15 ± 5 mm root length were selected and placed floating on well aerated solution, in a controlled water bath at 25º ± 1ºC, with a relative humi-dity of about 60% and irradiance intensity of 150 mmoles Q m-2s-1. Seedlings were grown for three days in a basal nutrient solution (modified from Camargo & Oliveira, 1981). The pH of the basal solution was set at 4.1 ± 0.1 and adjusted daily with 0.1 N HCl. After complete induction of nitrate and ammonium transport systems (Glass et al., 2002), the plants were transferred to different treatment solutions for four days. After treatment, the plants were transferred to the correspon-ding control treatment solution (without Al), added by 125 mM KH2PO4 and all micronutrients, and kept at constant pH 4.1 ± 0.1, for a recovery period of three days. The macronutrients composition of basal and ten treatment solutions is described in Table 1. The solutions were supplemented with micronutrients as follows: 7.5 mM NaCl; 2.5 mM H3BO3; 0.5 mM MnSO4.4H2O; 75 nM CuSO4; 200 nM ZnSO4; 25 nM Na2MoO4.2H2O; and 2.5 mM FeCl3.6H2O (Camargo & Oliveira, 1981). The ten treatment solutions differed mainly in NO3-/ NH4+ ratios (15:1, 8:1, 3:1 and 1:1) and were added or not by 370 mM Al, as Al2(SO4)3.16 H2O - A0 to E0 were control solutions without Al and treatment solutions A1 to E1 had Al added. All treatment solutions were set initially at pH 4.1 ± 0.1, they were supplemented with the micronutrients, except for iron, and were unprovided of phosphorus, to avoid Al precipitation. The pH was measured periodically.
Table 1 – Concentration of macronutrients, expressed in mM, in the basic and the ten treatment solutions, which were supplemented with micronutrients and had 0 mM (A0, B0, C0, D0, and E0) or 370 mM Aluminium (A1, B1, C1, D1, and E1).
The roots were measured with accuracy of 0.5 mm. Separately, shoots and roots were weighted, at the end of each growth period (after three, seven and ten days of assay). The relative reduction (RR) of root elongation or biomass increment of plants grown in a treatment solution was defined as the diffe-rence of the unit minus the ratio between the values obtained in Al treatment (x1) and those in the corresponding control (x0), expressed as percentage, i.e., RR = (1 - x1 / x0) 100. The net acquisition of NO3- and NH4+ from solutions was calculated by the depletion of these ions determined spectrophotometrically in solutions in the beginning and at end of each growing period, respectively, according to procedures adapted from Cataldo and collaborators (1975) and Solozano (1969).
There were two recipients with 90 plants in 3.8 litres of aerated basal solution. During the treatment and recovery periods there were, respectively, 12 and 6 plants, in pots with 300 ml of aerated solutions. The experiment included two factors, ratio of N mineral forms and Al doses, and three blocks (which had ten recipients completely randomised in each). ANOVA was applied and means compared by the Student test.
RESULTS
Root and shoot growth
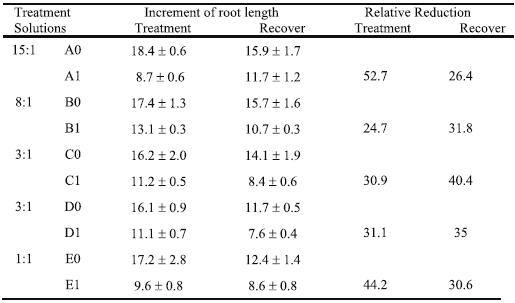
Table 2 shows the daily absolute increment of root length during four days of treatment with 0 or 370 mM Al. The increment of root length of young plants was not differently affected by N-form nutrition without Al, but under Al stress there were significant diffe-rences. So, the highest reduction relative to the corresponding control (52.7%) was observed in the solution with the 15:1 NO3-/ NH4+ ratio (A1). The Al presence in the solution with 1:1 NO3-/ NH4+ ratio (E1) induced a great reduction of daily root length increment also (44.2 %). Both solutions with 3:1 NO3-/ NH4+ ratio (C1 and D1) showed about one third of reduction relative to control and the lowest reduction (24.7%) was fund in the proportion 8:1 (B1). Unexpectedly, during the last three days of recovery, the plants grown with 3:1 (D0) and 1:1 (E0) NO3-/ NH4+ ratio evidenced significantly lower root elongation than the other control plants. The recovery of root elongation was reduced due to Al stress in all modalities.
Table 2 – Increment of root growth (mm day-1) and reduction relative to control (RR, %) of triticale TTE 9203 plants, during four days of treatment in solutions with different NO3-/ NH4+ ratios (as for Table 1), which were supplemented with 0 mM (A0, B0, C0, D0, and E0) or 370 mM Aluminium (A1, B1, C1, D1, and E1), and during three days of recovery in the respective control solution. Mean values ± standard error (n=3).
In the control solutions, the shoot biomass production with the 15:1 NO3-/ NH4+ ratio (A0) was higher than the observed for the 3:1 (C0 and D0) and 1:1 (E0) proportions. And the production of root biomass was significantly smaller with the 1:1 NO3-/ NH4+ ratio (E0) compared with other control solutions (Figure 1). After four days of treatment, the production of shoot biomass was not significantly affected by Al presence. But Al pre-sence affected negatively the root fresh weight in all modalities (Figure 1). The reductions relative to the corresponding controls were by decreasing order 40.6% (A1), 35.8% (E1), 24.2% (C1), 21.8% (D1), and 20.3% (B1).
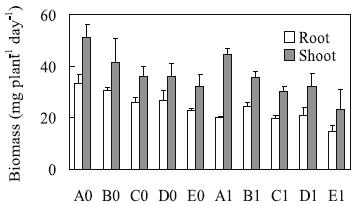
Figure 1 – Daily production of root and shoot fresh weight by triticale plants [mg fr wt (plant day)-1] grown in treatment solutions (as for Table 1), during four days. Means (n=3) ± standard error.
The ratio between the root and shoot fresh biomass of seven days old plants grown in solution with 15:1 NO3-/ NH4+ ratio decreased about 20% due to Al stress (from 0.655 to 0.523), whereas the other proportions induced insignificant changes of this parameter.
NO3- and NH4+ net acquisition
In control solution having the proportion 15:1 (A0), the NO3- depletion was almost complete (Table 3). Al stress reduced NO3- net uptake, but not NH4+ net acquisition by plants. The NO3- net acquisition was also limited by the higher NH4+ net acquisition (Table 3). The control plants grown with the proportions 3:1 and 1:1 of NO3-/ NH4+ ratio depleted NO3-, respectively, 60% (C0 or D0) and 68.7% (E0) of the availability in solutions. The Al treated plants depleted only 47.7% (C1 or D1) and 48.9% (E1) of the NO3- available in solutions in accordance with others (Antunes, 1998; Jerzykiewiez, 2001).
Table 3 – Nitrogen net acquisition during the four days of the treatment period (mmoles N plant-1 day-1), ratio between NO3- and NH4+ uptaken by plants, and N use efficiency (N U E, g fresh weight. mmole-1 N). Mean values ± standard error (n=3).
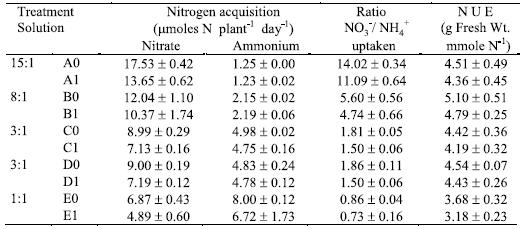
The ammonia depletion observed was almost complete in solutions with 15:1, 8:1 and 3:1 proportions (Table 3). In solutions with the 1:1 proportion (E0 and E1 with concentration of 1.6 mM NH4+-N), the plants had 10 mmoles NH4+-N plant-1 day-1 available inside each pot. The net acquisition of NH4+-N exceeded that of NO3--N, with and without Al stress. So, the ratio between NO3- and NH4+ uptaken by plants was lower than the unit (0.86 for E0 and 0.73 for E1).
The nitrogen use efficiency (NUE) corresponding to solution with 8:1 ratio were the highest. Whereas, the 1:1 solution ratio induced significantly lower NUE than the other proportions of NO3-/ NH4+, both with and without Al (Table 3). In all N-forms proportions, NUE was slightly affected by Al stress (Table 3).
Consistently, the presence of Al induced higher net acquisition of N per unit of root fresh weight, particularly in solutions with the proportions 15:1 and 1:1 (Figure 2). The NH4+-N uptake per unit of root fresh weight in the proportion 1:1 were the highest (see E0 and E1, in Figure 2), which might have induced NH4+ accumulation and toxicity in plant tissues.
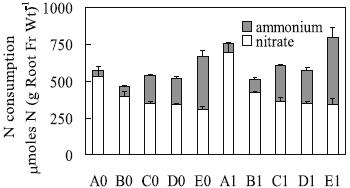
Figure 2 – Nitrate and ammonium net acquisition from different solutions (as for Table 1) per unit of root fresh weight by triticale plants [mmoles N (g root fr wt)-1] submitted to treatments during four days. Means (n=3) ± standard error.
Solution pH variation
In the first two days during the treatment period decreases of the pH values of 0.3 to 0.6 were observed in all the ten modalities. Thereafter, differential pH rises were observed in solutions containing different N-forms proportions or Al treatments. The control solutions with the proportions 15:1 and 8:1 changed to pH 6.0 and 5.8, respectively, while the other controls reached initial pH values of 4.0 ± 0.1, at the end of treatment period. In the presence of 370 mM Al, the solutions containing the proportions 15:1 and 8:1 reached also the initial pH values, but in those containing 3:1 and 1:1 null increases of pH could be detected in the last two days of treatment.
DISCUSSION
In the present experiment, the root length and root fresh biomass in 370 mM Al treatments showed more important reductions relative to controls in the plants grown in the root media with the highest NO3-/ NH4+ ratio (15:1), followed by the treatment with the highest NH4+ concentration (the 1:1 NO3-/ NH4+ ratio, containing 1.6 mM NH4+). Therefore, the Al toxicity to roots was smaller in the solutions containing the proportions 3:1 and, principally, 8:1 of NO3-/ NH4+ ratio. The effect of low ionic strength that enhanced the free Al ions activity observed by Pintro & Taylor (2004) could not explain the greater Al toxicity observed in our solutions A1 and E1 (see RR in Al treatment – Table 2). The electric conductivity of the control and 370 mM Al nutrient solutions rose gradually from A to E (as in Table 1), respectively, from 656 to 818 mS cm-1 and from 720 to 920 mS cm-1.
Previous results (Antunes, 1998) obtained with the Al-sensitive cv. Beagle treated with the proportions of 15:1 or 8:1 of NO3-/ NH4+ ratio, and 0 or 185 mM Al, also led to the conclusion that the young plants, once submitted to Al stress, grow better with enhanced ammonia nutrition with evidence for cation amelioration effect of NH4+ over Al toxic ions (Klotz & Horst, 1988).
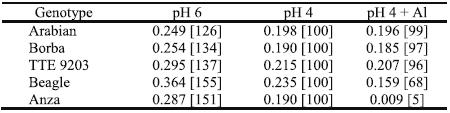
In an experiment with a basic diluted nutrient solution, containing 2,4 mM NO3- and 0,16 mM NH4+, with the pH fixed at 6,0 and 4,0 and, in this last modality with 0 or 185 mM Al added, there were very significant differences statistically (P= 0.05) among the three treatments for the growth triticale and wheat cultivars (Antunes, 1998). The seven days old plants of Beagle and principally Anza (Triticum aestivum) were H3O+- and Al-susceptible (Table 4) by the criteria of the relative elongation rate (RER) of the main root. The root elongation of young plants is considered one of the most sensitive indicators for plant proton and metals toxicities, in short term experiments. The roots elongation and their ability to explore water and nutrients in deeper soil layers are decisive to plant development, particularly to rain fed cereal crops. Potentially, the maintenance of high RER of plant roots in very acid root media added with Al toxic concentration might allow improved nutrition, long-term plant health and growth, and higher grain yields under acidic and Al-toxic soil conditions of the selected germplasm (Camargo & Oliveira, 1981; Antunes, 1998).
Table 4 –Relative Elongation Rate (mm mm-1 day-1) of roots of four triticale and one wheat genotypes during four days of treatment in solutions with pH 6.0 or 4.0 with 0 or 185 mM Aluminium (n=3). Between brackets are presented the percentage values relative to the treatment with pH 4 for each genotype.
In this triticale experiment, the pH reduction observed and the following pH increase were consistent with uptake from the nutrient solutions of NH4+ (phase I – external acidification) and of NO3- (phase II – external alkalisation), as stated by Taylor and Foy (1985). Indeed, the pH changes could be explained, at least partially (as other ions were also involved), by the N-ions influx by the roots. The passive influx of NH4+ by root cells is permitted by the efflux activity of proton pumps and NH4+ accumulation in tissues could be cytotoxic (Cruz, 1994). On the other hand, the uptake of NH4+ might inhibit the NO3- uptake, possibly because of the plasmalemma electrochemical potential disruption, which prevents the active symport of NO3- and H+ (Cruz, 1994). The measured NO3--N uptaken by the plants grown in the proportions 3:1 and, principally, 1:1, under Al stress, were the lowest, which might had turned unfeasible the rising of the low pH found the in these pots, during the last two days of treatment. Other authors suggested that the hydrolysis of NH4+-N could directly increase the concentration of H3O+ and indirectly raise the concentration of the Al soluble forms that induce higher rhizotoxicity (Andrade et al., 1996). This is a possibility, in the present conditions, at the highest NH4+ concentration (solution E1, with 1:1 ratio) with formation of H3O+ and free NH3, which would be phytotoxic (Cruz, 1994). Also, high NH4+ concentration could induce antagonism to other nutrient cations uptake by roots (Roy et al., 1988).
In all solutions, iron and phosphate were totally omitted during the treatment period to avoid the rapid precipitation of Al (Camargo & Oliveira, 1981). The presence the of highest sulphate concentration (900 mM SO42-) in solutions C1 and E1 could have induced Al chemical speciation with reduced rhizo-toxicity, but this effect was not observed. Although the enhanced sulphate could partially explain the slightly better recovery of the plants grown in solution C over the plants from D (both containing 3:1 NO3-/ NH4+ ratio).
The solutions D (with 3:1 NO3-/ NH4+ ratio) and E (1:1) had higher concentration of Cl- ions (respectively, 1415 and 2215 mM) in comparison with the other three solutions A (15 mM), B (165 mM) and C (615 mM). Britto and collaborators (2004) considered increased NaCl sensitivity and tissue Cl- accumulation under NH4+ nutrition. The recovery of root elongation of seven days old plants grown in control treatments D0 and E0 possibility indicates that the chloride might have exerted some interactions with the NO3- and NH4+ ions uptaken by the roots, in spite of the fact that the potentially toxic external level (100 mM Cl-) was not reached.
Many authors found reduced toxicity of Al organic complexes with carboxylic acids and phenols (Roy et al., 1988; Hue & Licudine, 1999), as well as of Al-sulphates and Al-phosphates in solutions (Mora et al., 2005). In fact, the manure, like other organic slud-ges and slurries, used as N source for plant nutrition (Yusuf et al., 2003), constitute an important valorisation of effluents of animal production. The organic fertilizers exhibit variable composition and unsteady NO3-/ NO3--N ratio (Van Kessell et al., 2000), due to their origin, storage and environmental conditions. They are also enriched or contain important amounts of compounds easily convertible into P2O5, K2O, and sulphate, that are available to plants. The surface applications of chicken manure and sewage sludge were superior to lime in increasing the soil pH and exchangeable Ca, and reducing Al toxicity, especially at lower soil depths (Hue & Licudine, 1999).
In soils with abundant water disposal (from rain or irrigation), ammonia is diluted and its toxicity to plants becomes limited or inexistent. On the other hand, cations in soil solution are less susceptible than anions of being dragged superficially or leached downward, as, the cation exchange pool of soil is adsorbed by negatively charged radicals of soil particles (Fernando et al., 2005). The N remaining in the top 60 cm of soils after fall-applied fertilisers on wheat were 9-20% for Ca(NO3)2 or 30-59% for (NH4)2SO4 (Huber et al., 1980). In Spring time, the greatest growths and biggest crop yields were obtained with (NH4)2SO4, especially when addicted with specific nitrification inhibitor (Huber et al., 1980). Naturally, under acidic or reducing conditions the nitrification of NH4+-N is low. To slow NO3--N release, a localised system of ammonia fertilisation, like band application, nests or large granules might be used, always preventing the risks of root or shoot direct damage (Shaviv, 2000). Alternatively, synchronisation of mixed N application with plant demand might be achieved by adding salts (stabilising and complementing the organic fertilisers with mineral nutrients) to the sludge applied directly on the soil to reduce NH3 volatilisation from and toxicity effects on the installed crop.
The amount and long distance transport of free reactive N species (like ammoniacal and nitric fertilizers not uptaken by plant roots) must be reduced in the environment. The peaks of NO3--N and NH4+-N uptake by the plant occurred in late afternoon, at the end of daylight hours (Glass et al., 2002). However, the night application of NH4+-N sources (after sunset and before sun rise), when the air temperature falls, under favou-rable weather (absence of wind) and mode-rate wet soil conditions, might be advisable to reduce volatilisation. Since the initial N losses by volatilisation are high in field crops, the recommended rates of organic NH4+-N sources applied to crops might be reduced. An effective agronomic useful use of N applied was achieved by intercropping rye cover with maize (Rasse et al., 2000). The circumscription of field plots with living borders, particularly of arboreal type (Preto, 1983), limits the long distance transport and the acidic deposition of nitrogen compounds else where, and reduces atmospheric acid pollution. This practice avoids the reduction of spontaneous plant diversity, which is susceptible to Al excess (Roem et al., 2002), specially the vanishing of slow growing plant species adapted to low N environment. Also, the deeper root systems permit to recycle N in percolating waters. The riverain vegetation might contribute to the depletion of soluble N lost downstream. The reduction of environmental pollution, and the increase or maintenance of NUE by applications of mineral, organic or bio-amended N-sources might be conjugated by the observation of proper agricultural techniques (Silgram & Shepherd, 1999; Kouyaté et al., 2000; Yusuf et al., 2003). Social and economical profits, and ecological conservation, by the maintenance of natural diversity of microorga-nisms, flora and fauna, might be satisfied in traditional/ or adapted integrated agro-ecosystem management (Preto, 1983).
Moreover, the plant breeding methods used to produce better cultivars might allow the conservation of the population genetic variability and plasticity (Ceccareli, 1996; Antunes, 1998), which are necessary to face crop constraints, like the soil vertical and horizontal heterogeneity, the seasonal and regional abiotic threats, the unpredictable climate change, and the old and emergent plant diseases and pests.
In many ecosystems N is the major limi-ting factor of productivity. The improper use of N fertilizers in agriculture can disrupt the global N cycle, and consequences might be dramatic, as the substancial acidification of soils, internal waters, and oceans, photochemical smog and increase of greenhouse gases (like N2O) in atmosphere. More than ever, the greatest attention applied to critical reductions of pollutants is needed to reach ecological equilibrium in this unique round earth and, also, an effective holistic perception of mankind as an important part of Nature (Rosen, 1970).
CONCLUSIONS
The following conclusions arise from the results: (1) the plants submitted to Al stress reached higher root length increment in solutions containing 8:1 and 3:1 (respectively with 0.350 and 0.800 mM NH4+, and 3.2 mM total-N available in nutrient solution) than in the solutions with 15:1 and 1:1 NO3-/ NH4+ ratio; (2) the presence of very toxic Al concentration affected less the biomass production in solutions having 8:1 and 3:1 NO3-/ NH4+ ratio; (3) the root medium pH was more acidic with enhanced NH4+-N nutrition, but under the present conditions, the NH4+ ions induced an amelioration effect of Al ions toxicity to this triticale genotype; (4) the presence of Al in solutions reduces significantly the nitrate acquisition per plant; (5) the highest plant N use efficiency also indicates the lowest Al toxicity in solution with 8:1 NO3-/ NH4+ ratio. Present results show that triticale TTE 9203 evidenced for good Al tolerance and, at early plant stages, it might benefit from mixed N inorganic fertilization in acid soils with Al toxic levels.
Acknowledgements
We present our highest esteem to the Professor Dra. Maria Antonieta Nunes who most honoured us with her scientific knowledge. This work stands as a homage to her proficient career in the Instituto de Investigação Científica Tropical. We thank Engº Benvindo Maçãs, from the Instituto Nacional de Recursos Biológicos, Estação Nacional de Melhoramento de Plantas (Elvas), who provided the triticale seeds, and Prof. Dra. Sara Amâncio, from the Universidade Técnica de Lisboa, Instituto Superior de Agronomia (Lisbon), who kindly reviewed this work. This work has been sponsored by the Fundação para a Ciência e Tecnologia.
BIBLIOGRAPHIC REFERENCES
Albassam, B.A. (2001) - Growth and nitrate assimilation in pearl millet exposed to aluminium stress. Saudi Journal of Biological Sciences 8: 105-112.
Alexander, K.G.; Miller, M.H. & Beauchamp, E.G. (1991) - The effect of an NH4+ enhanced nitrogen source on the growth and yield of hydroponically grown maize (Zea mays L.). Journal of Plant Nutrition 14: 31-44.
Andrade, L.R.M.; Ikeda, M. & Ishizuka, J. (1996) - Effect of nitrogen sources on aluminum toxicity in wheat varieties differing in tolerance to aluminum. Soil Science and Plant Nutrition 42: 651-657.
Aniol, A. (1996) - The variability of aluminium tolerance among triticale strains and cultivars bred in Poland. In: Guedes-Pinto, H.; Darvey, N. & Carnide, V.P. (Eds.) Triticale: today and tomorrow. Developments in plant breeding. Vol. 5, Kluwer Academic Publishers, Dordrecht, pp. 461-465.
Antunes, A.M. (1998) - Aspectos da tolerância ao alumínio em genótipos de triticale. Ph.D. thesis equivalent. Instituto de Investigação Científica Tropical, Lisboa, 181 pp.
Blamey, F.P.C.; Edwards, D.G. & Asher, C.J. (1983) - Effects of aluminium, OH:Al and P:Al molar ratios, ionic strength on soybean root elongation in solution culture. Soil Science 136: 197-207.
Bozzini, A. (1991) - The potential of triticale in Africa. Journal of Agriculture and Environment for International Development 85: 63-74.
Britto, D.T.; Ruth, T.J.; Lapi, S. & Kronzucker, H.J. (2004) - Cellular and whole-plant chloride dynamics in barley: insights into chloride-nitrogen interactions and salinity responses. Planta 218: 615-622. [ Links ]
Camargo, C.E.O. & Oliveira, O.F. (1981) - Tolerância de cultivares de trigo a diferentes níveis de alumínio em solução nutritiva e no solo. Bragantia 40: 21-31. [ Links ]
Cataldo, D.A.; Haroon, M.; Schrader, L.E. & Youngs, V.L. (1975) - Rapid colorimetric determination of nitrate in plant tissue by titration of salicylic acid. Communications in Soil Science and Plant Analysis 6: 71-80.
Ceccarelli, S. (1996) - Adaptation to low/ high input cultivation. Euphytica 92: 203-214.
Cruz, C.M.N. (1994) - Aspectos do metabolismo do azoto em plântulas de alfarrobeira (Ceratonia siliqua L.). Ph.D. thesis. Universidade de Lisboa/Faculdade de Ciências de Lisboa, Lisboa,199 pp.
Fernando, W.A.R.N.; Xia, K. & Rice, C.W. (2005) - Sorption and desorption of ammonium from liquid swine waste in soils. Soil Science Society of America Journal 69: 1057-1065.
Fields, S. (2004) - Global nitrogen cycling out of control. Environmental Health Perspectives 112: A557-A563.
Fleming, A.L. (1983) - Ammonium uptake by wheat varieties differing in Al tolerance. Agronomy Journal 75: 726-730.
Glass, A.D.M.; Britto, D.T.; Kaiser, B.N.; Kinghorn, J.R.; Kronzucker, H.J.; Kumar, A.; Okamoto, M.; Rawat, S.; Siddiqi, M.Y.; Unkles, S.E. & Vidmar, J.J. (2002) - The regulation of nitrate and ammonium transport systems in plants. Journal of Experimental Botany 53: 855-864.
Huber, D.M.; Warren, H.L.; Nelson, D.W.; Tsai, C.Y. & Shaner, G.E. (1980) - Response of winter wheat to inhibiting nitrification of fall-applied nitrogen. Agronomy Journal 72: 632-637.
Hue, N.V. & Licudine, D.L. (1999) - Amelioration of subsoil acidity through surface application of organic manures. Journal of Environmental Quality 28: 623-632.
Jerzykiewicz, J. (2001) - Aluminium effect on nitrate assimilation in cucumber (Cucumis sativus L.) roots. Acta Physiologiae Plantarum 23: 213-219.
Jones, D.L. & Willett, V.B. (2006) - Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biology and Biochemistry 38: 991-999.
Justino, G.C.; Cambraia, J.; Oliva, M.A. & Oliveira, J.A. (2006) - Absorção e redução de nitrato em duas cultivares de arroz na presença de alumínio. Pesquisa Agropecuária Brasileira 41: 1285-1290.
Klotz, F. & Horst, W.J. (1988) - Effect of ammonium-and nitrate-nitrogen nutrition on aluminium tolerance of soybean (Glycine max L.). Plant and Soil 111: 59-65.
Kouyaté, Z.; Franzluebbers, K.; Juo, A.S.R. & Hossner, L.R. (2000) - Tillage, crop residue, legume rotation, and green manure effects on sorghum and millet yields in the semiarid tropics of Mali. Plant and Soil 225: 141-151.
Malhi, S.S.; Nyborg, M.; Caldwell, C.D.; Hoyt, P.B. & Leitch, R.H. (1988) - Effect of ammonium and nitrate on growth and yield of barley on acid soils. Communications in Soil Science and Plant Analysis 19: 1049-1063.
Mora, M.L.; Demanet, R.; Vistoso, E. & Gallardo, F. (2005) - Influence of sulfate concentration in mineral solution on ryegrass grown at different pH and aluminum levels. Journal of Plant Nutrition 28: 1117-1132.
Pintro, J.C. & Taylor, G.J. (2004) - Effects of aluminium toxicity on wheat plants cultivated under conditions of varying ionic strength. Journal of Plant Nutrition 27: 907-919.
Preto, G. (1983) - Importanza e prospettive dell´agro-selvicoltura tropicale. Journal of Agriculture and Environment for International Development 77: 319-341.
Rasse, D.P.; Ritchie, J.T.; Peterson, W.R.; Wei, J. & Smucker, A.J.M. (2000) - Rye cover crop and nitrogen fertilization effects on nitrate leaching in inbred maize fields. Journal of Environmental Quality 29: 298-304.
Roem, W.J.; Klees, H. & Berendse, F. (2002) - Effects of nutrient addition and acidification on plant species diversity and seed germination in heathland. Journal of Applied Ecology 39: 937-948.
Rosen, W.G. (1970) - The environmental crisis: through a glass darkly. BioScience 20: 1209-1211 + 1216.
Rosenzweig, C. & Hillel, D. (2000) - Soils and global climate change: challenges and opportunities. Soil Science 165: 47-56.
Roy, A.K.; Sharma, A. & Talukder, G. (1988) - Some aspects of aluminum toxicity in plants. Botanical Reviews 54: 145-178.
Shaviv, A. (2000) - Advances in controlled-release fertilizers. Advances in Agronomy 71: 1-49.
Silgram, M. & Shepherd, M.A. (1999) - The effects of cultivation on soil nitrogen mineralization. Advances in Agronomy 65: 267-311
Soares, M.I.M. & Lewis, O.A.M. (1986) - An investigation into nitrogen assimilation and distribution in fruiting plants of barley (Hordeum vulgare L. cv. Clipper) in response to nitrate, ammonium and mixed nitrate and ammonium nutrition. New Phytologist 104: 385-393.
Solozano, L. (1969) - Determination of ammonium in natural waters by the phenol-hypochloride method. Limnology and Oceanography 14: 799-801.
Taylor, G.J. & Foy, C.D. (1985) - Mechanism of aluminum tolerance in Triticum aestivum L. (wheat). IV The role of ammonium and nitrate nutrition. Canadian Journal of Botany 63: 2181-2186.
Vaast, P.; Zasoski, R.J. & Bledsoe, C.S. (1998) - Effects of solution pH, temperature, nitrate/ammonium ratios, and inhibitors on ammonium and nitrate uptake by Arabica coffee in short-term solution culture. Journal of Plant Nutrition 21: 1551-1564.
Van Kessel, J. S.; Reeves III, J.B. & Meisinger, J.J. (2000) - Nitrogen and carbon mineralization of potential manure components. Journal of Environmental Quality 29: 1669-1677.
Yusuf, A.A.; Chude, V.O. & Janssen, B.H. (2003) - Substitution rates of N, P and K in farmyard manure on an Alfisol in Northern Guinea savanna of Nigeria. Journal of Agriculture and Environment for International Development 97: 93-105.
Recepção/Reception: 2010.01.14
Aceitação/Acception: 2010.02.02














