Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de Ciências Agrárias
Print version ISSN 0871-018X
Rev. de Ciências Agrárias vol.32 no.2 Lisboa Dec. 2009
Irradiation effects on meat: a review
Efeito da irradiação na carne: uma revisão
Fábio Costa Henry 1
ABSTRACT
Food irradiation is a process exposing food to ionizing radiations such as gamma rays emitted from the radioisotopes 60Co and 137Cs, or, high energy electrons and X-rays produced by machine sources. Irradiation can induce formation of isooctane-soluble carbonyl compounds in the lipid fraction and acid-soluble carbonyls in the protein fraction of meat. Increasing irradiation dose increases these compounds however, cooking reduces them. Among the volatile components, 1-heptene and 1-nonene are influenced most by irradiation dose, and aldehydes (propanal, pentanal, hexanal) are influenced most by packaging type (aerobiose vs vacuum). Sulfur-containing volatiles formed from sulfur-containing compounds (primarily amino acids) also contribute to irradiation odor. Reducing the temperature during the irradiation process reduces the effects on odor/flavor because free radical generation and dispersion are reduced. Ultimately, radiolysis of water into free radical species may be the initiators of both lipid oxidation breakdown products and sulfur-containing volatiles responsible for irradiation odor. Methods to decrease the detrimental effects of irradiation include oxygen exclusion (vacuum packaging), replacement with inert gases (nitrogen) and addition of protective agents (antioxidants).
RESUMO
A irradiação de alimentos é um processo que expõe o alimento às radiações ionizantes tais como os raios gama, emitidos pelos isótopos radioativos 60Co e 137Cs, ou, os elétrons de alta energia e os raios X. A irradiação pode induzir a formação de compostos de carbonil isooctano solúveis na fração do lipídio e carbonils solúveis nos ácidos da fração proteica da carne. A dose crescente da irradiação aumenta estes compostos, entretanto o cozimento pode reduzi-los. Entre os componentes voláteis, 1 heptene e 1 nonene são mais influenciados pela dose de radiação, e os aldeídos (propanal, pentanal, hexanal) são mais influenciados pelo tipo de embalagem (aerobiose vs vácuo). Os compostos voláteis sulfurosos formados a partir dos compostos sulfurosos (primeiramente amino ácidos) também contribuem para o odor do alimento irradiado. A redução da temperatura durante o processo da irradiação reduz os efeitos indesejáveis no odor/sabor, pois a geração e a dispersão de radicais livres são reduzidas. Finalmente, a radiólise da água pode ser o iniciador dos produtos de decomposição da oxidação dos lipídios e dos componentes voláteis responsáveis pelo odor do alimento irradiado. Os métodos utilizados para diminuir os efeitos prejudiciais da irradiação incluem a exclusão do oxigênio (embalagem a vácuo), a utilização de gases inertes (azoto) e a adição de agentes protetores (antioxidantes).
INTRODUCTION
Soon after the discovery of X-rays in 1896, they were used for sterilization, medical and dental diagnostics, and for treatment of disease. By 1905, patents had been filed in the US (Patent No. 788480) and in Great Britain (Brit. Patent No. 1609) to use irradiation to improve the condition of food. In 1955, the US Army Medical Department began to assess the whether commonly consumed foods be safely irradiated (Brewer, 2009). In 1980, the Food and Agriculture Organization of the United Nations, the International Atomic Energy Agency and the World Health Organization (FAO/IAEA/WHO) sated that:
The irradiation of any food commodity up to an overall average dose of 1 Mrad (10 kGy) presents no toxicological hazard and introduces no special nutritional or microbiological changes; hence toxicological testing of foods so treated is no longer required (WHO, 1981).
Subsequently, the FDA proposed that specified foods irradiated at dosages not exceeding 100 krad were to be considered safe. Based on the Food Additives Amendment of 1958 to the Federal Food, Drug, and Cosmetic Act of 1938, irradiation is regulated as a food additive. Information requirements for the FDA to establish safety (radiological, toxicological and microbiological) of particular irradiated products have been reviewed by Pauli & Tarantino (1995). Food processors must use FDA-approved sources defined in the Code of Federal Regulations CFR (21 CFR 179.26): gamma-rays from sealed 60Co or 137Cs units, accelerated electrons generated from machine sources (<10 MeV), or X-rays generated from machine sources (<5 MeV). Because irradiation-induced pathogen reduction is most effective if applied after packaging, the packaging materials must also be approved by the FDA as safe under the conditions of the process.
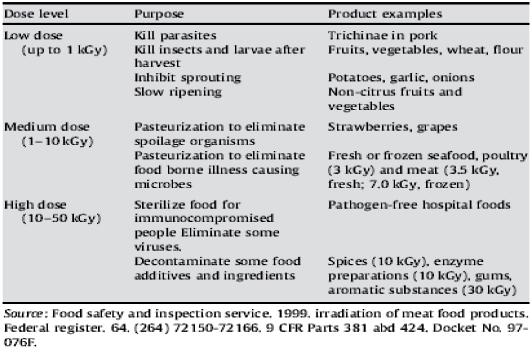
The maximum dose permitted for meat depends on the type (poultry vs red meat), and whether it is chilled or frozen. Irradiation of fresh and frozen poultry was approved in 1992; red meat irradiation was approved in 1997 (FSIS & Inspection Service, 1999). For pathogen reduction, a maximum of 4.5 kGy is permitted for uncooked, chilled red meat; 7.0 kGy is permitted for uncooked, frozen meat; 3.0 kGy is permitted for fresh or frozen poultry (Table 1).
Table 1 - Levels of irradiation permitted for food use
However, the detrimental effects of irradiation on odor, flavor and color have been major roadblocks for effective use of this technology to extend shelf life and reduce pathogen loads of fresh meat. In an effort to optimize conditions to procedure high quality products, the study of irradiation processing parameters has been under way for some time (Brewer, 2009).
THE IRRADIATION PROCESS
The process of irradiation has been reviewed by Brewer (2004; 2009) and Thakur & Singh (1994). Irradiation is electromagnetic energy which is the energy that holds atomic particles together. Change in the forces among atomic particles (protons, neutrons, electrons) results in destabilization of the atom. It re-stabilizes by emitting energy to re-balance the nucleus. Increasing the energy level of electrons leads to emission of that energy as the electrons return to their original energy levels. This electromagnetic energy and its emission are termed radiation. This radiation can take several forms depending on the level of energy being released. Sufficient energy to move the atoms in another molecule, but insufficient to change them chemically, is non-ionizing radiation. Sufficient energy to break chemical bonds is ionizing radiation – it has sufficient energy cause a particle (electron) to leave the atom producing an electron-deficient particle. The particle left behind has an imbalance between nuclear protons and orbital electrons. Low energy electromagnetic radiation occurs as very long wave lengths (microwave, radio waves); intermediate energy radiation occurs as visible light and heat; high energy radiation occurs as X-rays and Y-rays; very high energy radiation is emitted as radioisotopes decay (eg. Uranium; Fig. 1). High energy sources (X-rays, Y-rays, accelerated electrons) are ionizing; they can knock an electron from an atom creating ions or free radicals. Very high energy sources (radioactive substances) can split atoms, generate neutrons and produce radioactive by-products (Brewer, 2009).
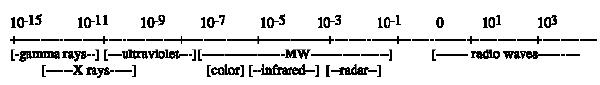
Fig. 1. - Electromagnetic spectrum-wavelength in meters (ICGFI, 1991)
The energy of Y-rays and X-rays can be transferred to other atomic particles. Y-rays do not ionize atoms directly but transfer energy to other atomic particles which interact with other materials to form ions. Y-rays can pass through living tissues without interacting with them because they are so high energy and so small. Y-rays are emitted from the nucleus of radioactive atoms; X-rays are emitted from the electron field (Efiok, 1996). Radionuclides approved for food irradiation, 137Cs and 60Co, produce Y-rays as they decay over time. They have short half lives (137Cs = 30 yr; 60Co = 5.2 yr) compared to radioisotopes (234Ur = 25,000 yr).
Irradiation dose is measured in Grays, the amount of energy per unit mass. The amount of energy to which a food is exposed is expressed as the radiation absorbed dose(rad). 1 kGy raises food temperature by <0.5oF.
1 Gray = 1 joule of energy absorbed/kg food
1 Gray = 6200 billion MeV absorbed/kg food
1 Gray = 100 Rads (or 0.022 calorie/kg of food)
1 Rad = 100 erg/g.
Because of the energy input, irradiation can induce reactions among food components including: oxidation of metals and ions, oxidation/reduction of carbonyls to/from hydroxy derivatives, elimination of unsaturation (double bonds), decrease in aromaticity, hydroxylation of aromatic and heterocyclics, and generation of free radicals which can be oxidized to various peroxides. These reactions can initiate lipid oxidation, break down of protein components, and damage vitamins, color, odor, and flavor (Brewer, 2009).
Sources of irradiation energy
The radionuclides approved for food irradiation include 137Cs and 60Co. Radioactive cobalt (60Co) decays to non-radioactive nickel by emitting a particles and γ-rays. The γ-rays kill rapidly growing cells (microbes) but do not leave the product radioactive (Lagunas-Solar, 1995). Because it is highly penetrating, it can be used to treat packaged food. Non-radioactive 137Cs occurs in various minerals. It can be produced when uranium and plutonium absorb neutrons and undergo fission in a nuclear reactor, then decay to non-radioactive barium by emitting ß particles and γ-rays (Brewer, 2009).
High energy particles can also be produced by accelerating electrons using electricity. These high energy, accelerated electrons are propelled out of an electron gun in a stream (e-beam). No radioactive source is required to produce accelerated electrons. They can penetrate 5-10 cm into food. X-rays can be produced by accelerating electrons into a thin metal plate (Satin, 2002).
Effect of fat content and lipid type
Irradiation results in volatile sulfur compounds responsible for the unique irradiation odor of ground beef. It also accelerates lipid oxidation on ground beef (Nam et al., 2003a). As far as the lipid fraction is concerned, unsaturated fatty acids are of primary concern. Because they are electron-deficient at the carbonyl groups and at the carbon double bonds, irradiation may result in formation of free radicals at these points (Thakur & Singh, 1994). Hydroxyl radicals (OH) tend to react with conjugated systems and are often considered to be the initiators of lipid oxidation in muscle tissue (Thakur & Singh, 1994). Autoxidation then proceeds via traditional pathways. The differents in total volatiles in irradiated pork patties has been reported to be due primarily to differences in aldehydes (Ahn et al., 1998a; Ahn et al., 1998b).
The volatile compounds responsible for the off-odor in irradiated meat produced by the impact of radiation on protein and lipid molecules are different from those of lipid oxidation alone (Jo & Ahn, 2000; Merritt et al., 1975). An increase in lipid peroxidation products (especially hexanal and trans-4, 5-epoxy-(E)-2-decenal) in combination with a loss of desirable meaty odorants (4-hydroxy-2, 5-dimethyl-3 (2H)-furanone and 3-hydroxy-4, 5-dimethy-2 (5H)-furanone) result in development of warmed over flavor of cooked, refrigerated beef (Kerler & Grosh, 1996). Du et al. (2001a) reported that irradiation of raw chicken breast produced alkanes and alkenes that appear to be result of both unsaturated fatty acid and amino acid breakdown.
Susceptibility of irradiated muscle tissues to lipid oxidation depends on endogenous characteristics of the tissue including the fat content, the fatty acid profile (proportion and degree of fatty acid unsaturation, and composition of phospholipids in the cell membrane) and the antioxidative potential of the tissue (Ahn et al., 1998b; Jo & Ahn, 2000). The most common fatty acids occurring in meat are oleic, linoleic, arachidonic, palmitc and stearic acids. Phospholipids constitute approximately 0.5-1% of the lean tissue (primarily phosphoglycerides) and a high proportion of unsaturated fatty acids that are susceptible to oxidation (Mottram, 1998). Phospholipids are also the source of several sulfides which are generated when they react with cysteine and/or ribose to produce mild, meaty beefy compounds (2-methyl-3[methylthio] thiophene) (Rowe, 2002).
Whether or not fat content per se affects irradiation-induced oxidative changes is unclear. In beef, increasing fat level (from 11 to 22%) has been shown to increase 2-butanone, 2-pentanone and 3-hydroxy-2-butanone (El Magoli et al., 1996). The effect of irradiation on volatiles varies among muscle types within species which may be a function of fat content of the respective muscles. Pork patties from L. dorsi muscle (>6.5% fat) had higher Thiobarbituric Acid Reactive Substances (TBARS) and contained more propanal and pentanal after irradiation than did those from the Psoas (1.8% fat) and R. femoris (2.4% fat) muscles (Ahn et al., 1998a). On the other hand, Houben et al. (2000) found no difference in TBARS on irradiated lean (<1% fat) versus higher fat (~20% fat) minced beef. It is likely that the unique fatty acid make-up of various meat species makes them more or less susceptible to irradiation-induced oxidation. Turkey and chicken dark meat contain similar amounts of linoleic acid (18:2; 1.75 and 1.87 g/100g lipid, respectively); (USDA National Nutrient Database for Standard Reference, 2007) and substantially more than is found in beef (0.12 g), pork (0.30 g), lamb (0.63 g), and Atlantic salmon (0.67 g). The total amount of 16:1, 18:1 and 18:2 is highest in chicken dark meat (5.33 g). It is similar in turkey white and dark meat, and Atlantic salmon (3.84, 3.34 and 1.82, respectively). These unsaturated fatty acids are primary source materials for lipid oxidation.
Autoxidation of linoleic acid produces, primarily, pentane, pentanal, hexanal, heptanal, (E)-2-heptanal, octanal, 1-octene, (z)-octanal, (E)-octanal, (E,Z)-2,4,-decadienal, wich produce grassy, rancid, fatty odors as well as lesser amounts 8-10 carbon aldehydes. The primary compound produced by autoxidation of linoleic acid is (E,Z)-2,4-heptadienal, which produces fishy, cooked meat odors, and lesser amounts of 2-10 carbon aldehydes. However, the odor thresholds of hexanal (1.7 µg; grassy, rancid) versus nonanal (13,500 µg; tallowy, fatty) make the relative contributions of these compounds to off-odors quite different (Belitz et al., 2004).
Because of the high concentration of dimethyl disulfide (cabage, putrid), 3-methylbutanal and 2-methylbutanal (rotten meat), and low concentrations of hexanal and pentanal (grassy, pungent), together with low TBARS, Du et al. (2002b) suggest that lipid oxidation immediately after irradiation is not the major contributor to off-odor of irradiated chicken.
Effect of antioxidants
Unless enzyme systems have been denatured, raw meat (unheated) has substantial antioxidant capacities (Ahn et al., 2000). Cooked meat is highly susceptible to oxidation because heat denatures antioxidant-forming systems and components, and damages cell structure exposing membrane lipids (phospholipids) to the environment (Ahn et al., 1998b). Antioxidants including free radicals scavengers (gallate, sesamol, tocopherol and butylated hydroxyanisole-BHA), metal chelators (ethylenediamine tetraacetic acid) and intrinsic antioxidants (carnosine) can reduce formation of off-odor volatiles in irradiated meat (Nam & Ahn, 2003).
Antioxidants can minimize irradiation-induced peroxidation of lard and tallow (Kyong et al., 1998; Kyong et al., 1999). Immediately after irradiation, ascorbyl palmitate (AP) was the most effective, followed by BHA, AP + α-tocopherol, and a-tocopherol alone. During storage, BHA was the most effective, followed closely by AP + α-tocopherol. AP was extremely effective in minimizing irradiation-induced oxidation in tallow, lard, and linoleic acid in a concentration-dependent fashion (Lee et al., 1999). Steady-state kinetic studies indicated that AP reduced oxidation by the oxygen-quenching mechanism. Adding ascorbic acid (0.1%) or sesamol + tocopherol (0.01%) to ground beef prior to irradiation can reduce generation of S-containing compounds (Nam et al., 2003a).
In some studies, inclusion of vitamin E (α-tocopherol) into animal diets has been shown to protect against lipid oxidation when meat is held in an oxygen-containing environment (Ahn et al., 1995; Houben et al., 2000). Vitamin E is retained in the membranes, in close proximity to phospholipids. Ahn et al. (1998c) reported that TBARS in irradiated turkey breast gradually decreased as dietary tocopherol acetate level increased.
Nam et al. (2003b) reported that these effects were dose-dependent. Dietary vitamin E protected irradiated, aerobically stored turkey meat against oxidative deterioration. The protective effect was dose-dependent; 100 IU vitamin E/kg diet was required to significantly reduce lipid oxidation. Similar findings associated with the natural vitamin E content of the lipid added to raw and cooked pork sausage (Jo & Ahn, 2000). However, Ohene-Adjei et al. (2004) reported that vitamin E-supplementation of swine diets had no effects on off-flavor of irradiated pork, initially or during retail display. Galvin et al. (1998) reported that supplementation with 200 mg α-tocopherol decreased TBARS formation rate. Authors concluded that, in general, irradiation has little effect on lipid stability in a-tocopherol supplemented chicken following cooking and storage. Ahn et al. (1998c) reported that α-tocopherol supplementation decreased hexanal production in irradiated, stored turkey in a dose-dependent fashion. While dietary vitamin E can control lipid oxidation on irradiated raw turkey, Ahn et al. (1998c) reported that it was insufficient for oxidation control in irradiated, cooked meat during storage (aerobic packaging).
Dietary conjugated linoleic acid (CLA) has been shown to reduce TBARS, hexanal and pentanal formation in irradiated raw chicken (Du et al., 2000; Wiegand et al., 2001). It decreased TBARS, 18:3, and 18:1, and increased 18:0 and t, t-9, 11-isomers in the fat of irradiated beef patties derived from steers fed CLA (Wiegand et al., 2001). However, it was ineffective in maintaining volatile profiles (Du et al., 2001a; Du et al., 2001b; Du et al., 2002a). Irradiation accelerated lipid oxidation in aerobically packaged cooked chicken, but its effect was not as significant as that of packaging. Vacuum packaging almost completely protected lipids from oxidation and dramatically reduced volatile production in both irradiated and non-irradiated raw chicken samples (Du et al., 2000).
Added sesamol, quercetin and BHT are effective in controlling oxidation in both irradiated raw and cooked pork during storage (Chen et al., 1999). Sesamol + α-tocopherol (0.02%) added to pork patties prior to irradiation (4.5 kGy) has been shown to reduce TBARS in aerobically packaged pork during storage (Nam et al., 2002). Irradiation produced significant amounts of sulfur volatiles under vacuum packaging conditions. Propyl gallate + α-tocopherol reduced dimethyl disilfide and dimethyltrisulfide formation by > 50% and > 90%, respectiveliy, compared to the irradiated control (no antioxidant). These compounds disappeared after storage in aerobic conditions. Nam & Ahn (2003) reported that the antioxidant-induced reduction of both lipid oxidation and production of sulfur volatiles was more apparent in vacuum packaged than in aerobically packaged pork. Of the antioxidants evaluated, sesamol was most effective, reducing dimethyl disulfide and total volatiles by 48% and 43%, respectively.
REFERENCES
Ahn, D. U., Jo, C., Du, T., Olson, D. G. & Nam, K. C. (2000) - Quality characteristics of pork patties irradiated and stored in different packaging and storage conditions. Meat Science 56, 2: 203–209.
Ahn, D. U., Kawamoto, C., Wolfe, F. H. & Sim, J. S. (1995) - Dietary alpha-linolenic acid and mixed tocopherols, and packaging influence lipid stability in broiler chicken breast and leg muscle tissue. Journal of Food Science 60: 1013–1018.
Ahn, D. U., Olson, D. G., Jo, C., Chen, X., Wu, C. & Lee, J. I. (1998a) -Effect of muscle type, packaging and irradiation on lipid oxidation volatile production and color in raw pork patties. Meat Science 49, 1: 27–39.
Ahn, D. U., Olson, D. G., Lee, J. I., Jo, C., Wu, C. & Chen, S. (1998b) -Packaging and irradiation effects on lipid oxidation and volatiles in pork patties. Journal of Food Science 63, 1: 15–19.
Ahn, D. U., Sell, J. L., Jo, C., Chen, X., Wu, C. & Lee, J. I. (1998c) - Effects of dietary vitamin E supplementation on lipid oxidation and volatiles content of irradiated cooked turkey meat patties with different packaging. Poultry Science 77: 912–920.
Belitz, H. D., Grosch, W. & Schieberle, P. (2004). Activated oxygen. In Food Chemistry. Berlin: Springer, pp. 200–208.
Brewer, M. S. (2004) -Irradiation effects on meat color–a review. Meat Science 68: 1–17.
Brewer, M. S. (2009) - Irradiation effects on meat flavor-a review. Meat Science 81: 1-14. [ Links ]
Chen, X., Jo, C., Lee, J. I. & Ahn, D. U. (1999) -Lipid oxidation, volatiles and color changes of irradiated pork patties as affected by antioxidants. Journal of Food Science 64, 1: 16–19.
Du, M., Ahn, D. U., Nam, K. C. & Sell, J. L. (2000) - Influence of dietary conjugated linoleic acid on volatile profiles, color and lipid oxidation of irradiated raw chicken meat. Meat Science 56, 4: 387–395.
Du, M., Hur, S. J., Nam, K. C., Ismail, H. & Ahn, D. U. (2001a) - Volatiles, color, and lipid oxidation of broiler breast lets irradiated before and after cooking. Poultry Science 80, 12: 1748–1753.
Du, M., Ahn, D. U., Nam, K. C. & Sell, J. L. (2001b) - Volatile profiles and lipid oxidation of irradiated cooked chicken meat from laying hens fed diets containing conjugated linoleic acid. Poultry Science 80, 2: 235–241.
Du, M., Nam, K. C., Hur, S. J., Ismail, H. & Ahn, D. U. (2002a) -Effect of dietary conjugated linoleic acid, irradiation, and packaging conditions on the quality characteristics of raw broiler breast lets. Meat Science 60: 9–15.
Du, M., Hur, S. J. & Ahn, D. U. (2002b) -Raw-meat packaging and storage affect the color and odor of irradiated broiler breast lets after cooking. Meat Science 61: 49–54.
Efiok, B. J. S. (1996) -Basic calculations for chemical and biological analyses. Gaithersburg, MD: AOAC International, pp. 84–86.
El Magoli, S. B., Laroia, S. & Hansen, P. M. T. (1996) - Flavor and texture characteristics of low fat ground beef patties formulated with whey protein concentrate. Meat Science 42: 179–193.
FSIS, Food Safety and Inspection Service. (1999) -Irradiation of Meat Food Products. Federal Register 64. (264) 7215072166. 9 CFR Parts 381 abd 424. Docket No. 97-076F.
Galvin, K., Morrissey, P. A. & Buckley, D. J. (1998) - Effect of dietary alpha-tocopherol supplementation and gamma-irradiation on alpha-tocopherol retention and lipid oxidation in cooked minced chicken. Food Chemistry 62, 2: 185–190.
Houben, J. H., van Dijk, A., Eikelenboom, G. & Hoving-Bolink, A. H. (2000) - Effect of vitamin E supplementation, fat level and packaging on color stability and lipid oxidation in minced beef. Meat Science 55: 331–336.
Jo, C. & Ahn, D. U. (2000) - Volatiles and oxidative changes in irradiated pork sausage with different fatty acid composition and tocopherol content. Journal of Food Science 65, 2: 270–275.
Kerler, J. & Grosch, W. (1996) -Odorants contributing to warmed-over flavor (WOF) of refrigerated cooked beef. Journal Food Science 61, 6: 1271–1274.
Kyong, H. L., Hong, S. Y., Ju, W., Hyun, J. L. J. K. & Myung, B. (1998) - Effects of antioxidants on oxidation of lard by gamma irradiation. Korean Journal of Food Science and Technology 27, 6: 1047–1052.
Kyong, H. L., Hong, S. Y., Ju, W., Sung, M. J. K. & Myung, B. (1999) - Effects of antioxidants on oxidation of tallow by gamma irradiation. Korean Journal of Food Science and Technology 31, 1: 7–12.
Lagunas-Solar, M. C. (1995) - Radiation processing of foods: An overview of scientific principles and current status. Journal of Food Protection 58, 2: 186–192.
Lee, K. H., Yook, H. S., Lee, K. W., Park, W. J., Kim, KS. & Byun, M. W. (1999) -Quenching mechanism and kinetics of ascorbyl palmitate for the reduction of the gamma irradiation- induced oxidation of oils. Journal of the American Oil Chemists Society 76, 8: 921–925.
Merritt, C., Jr., Angelini, P., Wierbicki, E. & Shultz, G. W. (1975) - Chemical changes associated with flavor in irradiated meat. Journal of Agriculture and Food Chemistry 23: 1037–1041.
Mottram, D. S. (1998) -Flavour formation in meat and meat products: a review. Food Chemistry 62: 415–424.
Nam, K. C. & Ahn, D. U. (2003) -Use of antioxidants to reduce lipid oxidation and off-odor volatiles of irradiated pork homogenates and patties. Meat Science 63, 1: 1–8.
Nam, K. C., Min, B. R., Park, K. S., Lee, S. C. & Ahn, D. U. (2003a) -Effects of ascorbic acid and antioxidants on the lipid oxidation and volatiles of irradiated ground beef. Journal of Food Science 68, 5: 1680–1685.
Nam, K. C., Min, B. R., Yan, H., Lee, E. J., Mendonca, A. & Wesley, I. (2003b) -Effect of dietary vitamin E and irradiation on lipid oxidation, color, and volatiles of fresh and previously frozen turkey breast patties. Meat Science 65, 1: 513–521.
Nam, K.C., Prusa, K.J. & Ahn, D.U. (2002) -Addition of antioxidant to improve quality and sensory characteristics of irradiated pork patties. In: Proceeding of the Annual Meeting of the Institute of Food Technologists. Anaheim, CA. Paper 88-7.
Ohene-Adjei, S., Bertol, T., Hyung, Y., Ellis, M., McKeith, F. K. & Brewer, M. S. (2004). Effect of vitamin E, low dose irradiation and display time on the quality of pork. Meat Science 68: 19–26.
Pauli, G. H. & Tarantino, L. M. (1995) - FDA regulatory aspects of food irradiation. Journal of Food Protection 58: 209–212.
Rowe, D. (2002). High impact aroma chemicals. Part 2: the good, the bad, and the ugly. Perfumer and Flavorist 27: 24–29.
Satin, M. (2002). Use of irradiation for microbial decontamination of meat: situation and perspectives. Meat Science 62: 277–283.
Thakur, B. R. & Singh, R. K. (1994) - Food irradiation Chemistry and applications. Food Reviews International 10, 4: 437– 473.
USDA National Nutrient Database for Standard Reference, Release 20. (2007) -http://www.nal.usda.gov/fnic/foodcomp/cgi-bin/ WHO. (1981). Wholesomeness of Irradiated Foods. World Health Organization. Technical Report Series 659. Geneva.
Wiegand, B.R., Gassman, K., Swan, J.E., Parrish Jr., F.C., Trenkle, A.H. & Larsen, S.T. (2001) -Conjugated linoleic acid (CLA) in diets of beef steers results in higher red fat oxidation and flavor, color and vitamin changes in meat. Quartermaster Food color scores and lower lipid oxidation of irradiated ground beef patties. Beef Research Report, Iowa State University, Ames, IA. A.S. Lea.et R1764.
1 Laboratório de Tecnologia de Alimentos (LTA), Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF), CEP 28013-602, Campos dos Goytacazes, RJ, Brasil. e-mail: fabiocosta@uenf.br
Recepção/Reception: 2009.06.14
Aceitação/Acception: 2009.08.14














