Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Portuguesa de Saúde Pública
versão impressa ISSN 0870-9025
Rev. Port. Sau. Pub. vol.33 no.1 Lisboa jun. 2015
https://doi.org/10.1016/j.rpsp.2014.07.005
ORIGINAL ARTICLE
Comorbidities and medication intake among people with dementia living in long-term care facilities
Comorbilidades e medicação em pessoas com demência em lares de idosos
Alda Marquesa, b, *, , Vânia Rochaa, , Margarida Pintoa, , Liliana Sousab, c, , Daniela Figueiredoa, b,
a School of Health Sciences, University of Aveiro (ESSUA), Aveiro, Portugal
b Cintesis.UA (Center for Health Technology and Services Research), University of Aveiro, Aveiro, Portugal
c Department of Health Sciences (SACS), University of Aveiro, Aveiro, Portugal
ABSTRACT
Information on comorbidities and medication of people with dementia (PWD) in long-term-facilities (LTF) is scarce. This study analysed type and number of comorbidities and medication of PWD in LTF. A descriptive-study was conducted in 40 LTF, characterising 329 PWD. Socio-demographics, dementia type, comorbidities and medication were collected with International-Classification-of-Functionality-checklist. Cognitive impairment was assessed with Mini-Mental-State-Examination. One or more comorbidities (2.1 ± 1.6) were found in 271 participants. Hypertension, osteoarticular-problems, heart-disease and type-II-diabetes were the frequently comorbidities. 327 participants consumed one or more medicines (7.3 ± 3.2), mainly for cardiovascular system, anxiolytics and antipsychotics. Comorbidities and medication amount was significantly different among cognitive impairment levels. Vascular comorbidities were present in all dementia types.
Keywords: People with dementia. Comorbidities. Medication. Long-term care facilities.
RESUMO
O conhecimento acerca das comorbilidades e da medicação em pessoas com demência (PCD) que vivem em lares de idosos é limitado. Assim, este estudo analisou o tipo e número de comorbilidades e medicação de PCD que vivem em lares de idosos portugueses. Um estudo descritivo foi conduzido em 40 em lares de idosos. Foram incluídas 329 pessoas com diagnóstico de demência, das quais foram recolhidas informações sociodemográficas, das comorbilidades e medicação com a checklist da Classificação Internacional de Funcionalidade. O défice cognitivo foi avaliado através do Mini-Mental State Examination. Uma ou mais comorbilidades (2,1 ± 1,6) foi encontrada em 271 participantes. Hipertensão, problemas osteoarticulares, doença cardíaca e diabetes tipo II foram as comorbilidades mais frequentes. 327 participantes consumiam um ou mais medicamentos (7,3 ± 3,2), principamente medicação para o sistema cardiovascular, ansiolíticos e anti-psicóticos. A quantidade de comorbilidades e medicação foi significativamente diferente entre os níveis de défice cognitivo. Comorbilidades vasculares estavam presentes em todos os tipos de demência.
Palavras-chave: Pessoas com demência. Comorbilidades. Medicação. Lares de idosos.
Introduction
Dementia is a global health challenge.1 In the year 2010 it was estimated that 35.6 million people had Alzheimer's disease and other dementias worldwide.2 This number will increase with an ageing world population and will reach 66 million by the year 2030 and 115 million by 2050.1
Comorbidities are highly prevalent among people with dementia3 and have been reported as risk factors for cognitive impairment and dementia progression. A study associated comorbidities to half of the Alzheimer's disease cases worldwide and it has been suggested that the reduction of 10–25% in these factors could prevent 3 million cases of Alzheimer's disease worldwide.4 Consequently, comorbidities contributes to dementia onset4 and may also lead to faster progression of the disease, representing an additional factor for disability and increased costs.5 However, the levels of comorbidities in people with dementia remain a controversial issue, with some studies reporting that this population present fewer than non-demented people6,7 and others suggesting more comorbidities than generally thought.8,9 This controversy causes difficulties when appropriate measures to prevent and treat comorbidities in dementia have to be defined.10 Additionally, high levels of comorbidities have been linked with high amount of prescribed medication in people with dementia when compared with other individuals.11 The use of psychotropic medication (antipsychotics, antidepressants and anxiolytics) in people with dementia has been studied.11 However, this medication, consumed specially by people living in long-term care facilities, showed to be inappropriate and has been associated with numerous adverse events, which led to hospitalisation and higher mortality.11 Nevertheless, the overall medication description in people with dementia living in long-term care facilities10,12 is still unknown, as this population due to their cognitive impairment tend to be excluded from the studies. In a time in which reducing costs and optimising health care is crucial,2 characterising comorbidities and medication intake of people with dementia becomes crucial, as this information has the potential to inform decision on appropriate strategies to prevent and treat comorbidities, adjust medication and support planning of health and social resources.13 Therefore, this study aimed to analyse the type and number of comorbidities and medication of people with dementia living in long-term care facilities.
Methods
Study design
An exploratory descriptive study was conducted in the central region of Portugal. The study was submitted to the Ethics Committee of the Research Unit of Health Sciences at the Health School of Nursing in Coimbra (UICISA: E), Portugal (Ref. 5-11/2010) and approval was obtained. Legal representatives were invited to attend a meeting where verbal and written information about the study was provided. A brief explanation about the study was also given to people with dementia. Written informed consents were collected from the legal representatives of the people with dementia living in the long-term care facilities prior to any data collection.
Participants
Fifty-seven long-term care facilities were contacted and information about the study was provided to the service managers in an arranged meeting. Forty care facilities with a total of 1780 residents accepted to participate. Participants were included in the study if they presented a medical diagnosis of irreversible dementia according to the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) criteria.14 People with dementia were excluded if: (i) refused to answer to the Mini-Mental State Examination (MMSE); (ii) were severe or total sightless and/or severe hearing impaired; (iii) had not been at the care facility for at least 3 months (considered the minimum individual time needed to adjust to the institution dynamics); (iv) did not have a legal representative to sign the written informed consent; (v) or died during the data collection.
From the total individuals living in the long-term care facilities, 353 (19.8%) had a medical diagnosis of dementia. However, 24 subjects were excluded as they: (i) refused to answer to the MMSE (n = 4) or their family did not sign the written informed consent (n = 2); (ii) were severe or total sightless (n = 4) and/or severe hearing impaired (n = 2); (iii) have not been at the care facility for at least 3 months (n = 6); (v) or died during the data collection (n = 10). Thus, a total of 329 people with dementia were included.
Measures
A structured questionnaire based on the International Classification of Functionality, Disability and Health (ICF) checklist15 was used to collect data about socio-demographics, type of dementia, type and number of comorbidities and medication of people with dementia. This is a checklist of major categories of the International Classification of Functioning, Disability and Health (ICF) of the World Health Organization. It is a practical tool to record health information of each individual and across different populations. This information was obtained from clinical files and staff (health professionals, service managers, direct care providers).
The MMSE adapted to the Portuguese population16 was applied to the people with dementia to assess their cognitive status. The severity of the cognitive impairment was characterised using the MMSE cut-offs, published in European studies, i.e., 21–27 mild, 11–20 moderate and 0–10 severe.
Data analyses
Data analysis was performed using the PASW Statistics version 18.0 for Windows (SPSS Inc., Chicago, IL). Descriptive statistics were applied to characterise the sample. Non parametric tests were performed as variables did not follow a normal distribution and the sample size was lower than 30 subjects in some variables sub-groups.17 Therefore, the Mann–Whitney and Kruskal–Wallis non-parametric tests were applied to explore the differences between the number of comorbidities and the sample characteristics (age, education, marital status, period living in the long-term care facility, type of dementia and severity of cognitive impairment); and the amount of medication used and the sample characteristics. The correlation between the number of comorbidities and the amount of medication used was analysed with the Pearson coefficient (r), as the sample size was higher than 30 subjects.17 A ρ-value less than 0.05 was considered statistically significant.
Results
Sample characteristics
Table 1 describes the sample characteristics. People with dementia mean age was 83.6 ± 7.1 years old. Most were older than 85 years old (n = 158; 48%), female (n = 262; 79.6%), had one to four years of education (n = 168; 51.1%) or no education (n = 97; 29.5%), were widowed (n = 200; 60.8%) and were living in the facility for less than 1 year (n = 105; 31.9%) or from 2 to 4 years (n = 119; 36.2%). Most participants did not have their type of dementia defined (n = 148; 45%) however, from the participants who presented a specific diagnosis, Alzheimer's disease was the most prevalent (n = 138; 41.9%). According to the MMSE, an average score of 8.7 ± 7.9 was found and 61.7% (n = 203) had severe cognitive impairment (Table 1).
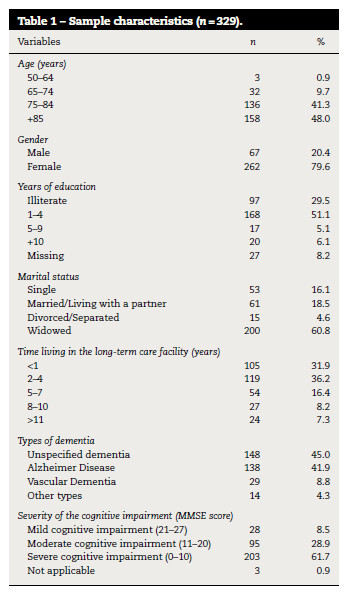
Comorbidities
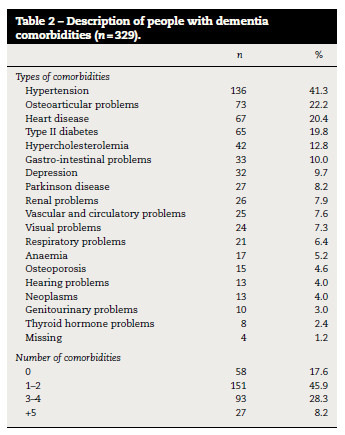
One or more comorbidities (2.1 ± 1.6) were found in 271 (82.4%) participants (Table 1). The most frequent comorbidities were hypertension (n = 136; 41.3%), osteoarticular problems (n = 73; 22.2%), heart disease (n = 67; 20.4%), type II diabetes (n = 65; 19.8%) and hypercholesterolemia (n = 42; 12.8%) (Table 2).
Most participants with unspecified dementia (n = 67) and Alzheimer's disease (n = 71) had 1–2 comorbidities, whilst subjects with vascular dementia (n = 12) had 3–4 comorbidities (table 3). Vascular risk factors were the most frequent among participants however, they were more prevalent in people with vascular dementia (hypertension – 68.9%, heart disease – 44.8%, type II diabetes – 37.9% and hypercholesterolemia – 24.1%) than in people with unspecified dementia (hypertension – 40.5%, heart disease – 22.9%, type II diabetes – 17.2% and hypercholesterolemia – 12.2%) or Alzheimer disease participants (hypertension – 35.8%, heart disease – 13.4%, type II diabetes – 17.2% and hypercholesterolemia – 10.4%).
The number of comorbidities was not significantly different according to age or gender however, it was significantly different among the types of dementia (ρ < 0.001) and severity (ρ = 0.005) of cognitive impairment (table 3), in which individuals with severe cognitive impairment presented a higher amount of comorbidities than those with mild to moderate cognitive impairment. However, 14.0% (n = 46) of the sample with severe cognitive impairment also presented no comorbidities (table 3).
Medication intake
One or more medicines (7.3 ± 3.2) were consumed by 327 (99.4%) participants (Table 4). Medication for the cardiovascular system (n = 238; 72.3%), anxiolytics (n = 178; 54.1%) and antipsychotics (n = 176; 53.5%) were the most commonly used (Table 4).
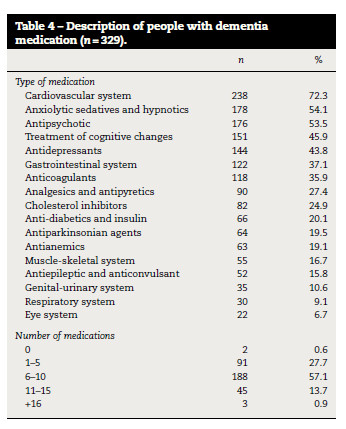
There were no statistically significant differences between the amount of medication used and age, gender and type of dementia (table 3). The amount of medication was significantly different among the levels of cognitive impairment (ρ < 0.001) (table 3) i.e., a higher amount of medication was observed in people with severe cognitive impairment.
Number of comorbidities vs. amount of medication
There was a significant positive correlation of 0.39 (ρ < 0.001) between the number of comorbidities and the amount of medication used by people with dementia living in long-term care facilities.
Discussion
Similarly to others, this study found a high number of comorbidities in people with dementia living in long-term care facilities, specifically hypertension,18 heart disease,10 type II diabetes19 and hypercholesterolemia.18 These comorbidities, which represent vascular risk factors, have been referred as highly prevalent in numerous populations with dementia8,20 and have been associated with an increased risk of developing dementia.21 Evidence4,22 suggests that preventing and treating vascular risks factors could prevent or delay the increment of cognitive impairment in people without dementia and reduce the burden associated with vascular risk factors on those with dementia. Therefore, as no cure is available for dementia, primary prevention of comorbidities, seems to have great potential to reduce the number of dementia cases emerging each year4 however, this needs further investigation.
Although the number of comorbidities was not significantly different considering age and gender,8 people with more severe cognitive impairment had a higher number of comorbidities. Moreover, the absence of comorbidities in several individuals with severe cognitive impairment, found in this study, suggests a possible lack of diagnosis.23,24 This means that if the comorbidities diagnosis is not performed in mild to moderate stages, it might be difficult to be made in the severe stages of the disease, since this population does not complain or receive frequent clinical revision.23
Furthermore, the amount of comorbidities was significantly different according to type of dementia. Therefore, vascular dementia was associated with a higher number of comorbidities than unspecified dementia or Alzheimer's disease.8,25 This type of dementia had shown a higher prevalence of vascular risk factors, such as hypertension, heart disease, type II diabetes and hypercholesterolemia. Although Alzheimer's disease and vascular dementia shared several risk factors, hypotheses have been raised suggesting that the increase burden associated with vascular risk factors or with the number of vascular diseases lead to an increased risk of vascular dementia,26 explaining the findings of this study.
The average number of medication prescribed to people with dementia living in long-term care facilities was 7.3 ± 3.2, higher than the average found in other research studies which ranged between 5.1 and 6.53,27,28 however, similar to the study of Andersen et al.,10 that reported a mean of 6.9 ± 3.9 medicines. The medication used in dementia has been associated with several side effects, e.g., deterioration of cognitive and psychomotor function, sedation, falls, fractures,29 increased risk of delirium,11 increased risk of stroke and dying.30 Therefore, the adequacy of the medication in people with dementia should be explored for each person before decisions are taken 30 since it appropriate use has already been questioned,10–12 mainly in long-term care facilities.31 Furthermore, non-pharmacological interventions seem a promising approach to reduce these type of medication32 and merits further research.
Previous studies reported that people with more severe cognitive impairment consume a higher amount of medication,10,33 which is in agreement with the results of this study. Questions on the potential overuse and cost-effectiveness of medication in people with dementia should be assessed in all stages of dementia and mainly on severe dementia, in which decisions to discontinue some medication should be explored, e.g., antipsychotics interruption if symptoms have been absent or minimal for three months.12
The significant positive correlation found between the number of comorbidities and the amount of medication prescribed in people with dementia was previously reported by Linjakumpu et al.,34 and highlights the importance of preventing and treating comorbidities to reduce the use of medication in this population. A lower number of comorbidities and consequently amount of medication would potentially result in the reduction of cognitive and functional decline, lower risk of institutionalisation, hospitalisation3 and mortality and therefore, merits further research.
Clinical implications
-
High levels of comorbidities (i.e., hypertension, osteoarticular problems, heart disease, type II diabetes and hypercholesterolemia) were found in people with dementia living in long-term care facilities. The supervision and earlier treatment of these comorbidities might reduce morbidity and improve quality of life of people with dementia.
-
A large amount of medication was consumed by people with dementia in all stages of the disease, mainly medication for the cardiovascular system, anxiolytics and antipsychotics.
-
Vascular dementia was associated with a higher number of comorbidities than unspecified dementia or Alzheimer's disease. Treating vascular risk factors might be important to reduce the risk of vascular dementia.
Conclusion
The high number of comorbidities found, mainly vascular risk factors for dementia onset and progression, raises awareness for the potential prevention of dementia cases through early diagnosis and treatment of vascular risk factors and comorbidities.
A large amount of medication in people with dementia was also found, when compared with previous studies. This study alerts for the possibility of inappropriate use of medication in people with dementia living in long-term care facilities. Additionally, as the number of comorbidities was linked with the amount of medication, the prevention and treatment of comorbidities could result in a lower amount of medication intake.
The design of this study was suitable to perform a descriptive analysis on comorbidities and medication intake of Portuguese people with dementia living in log-term care facilities. However, comparisons across studies using different methodologies impair the generalisation of results. Future research should be conducted with more robust designs, including a control group of people with dementia living in the community should be conducted to strengthen these findings and explore differences thoroughly.
Since a cure for dementia is not available yet, finding effective approaches to reduce the burden associated with the disease, improve people's quality of life and reduce the disease associated costs are essential for a sustainable society in an aging world.
References
1 Wortmann M. Dementia: A global health priority: Highlights from an ADI and World Health Organization report. Alzheimers Res Ther. 2012;4:40. [ Links ]
2 Association Alzheimer's 2012 Alzheimer's disease facts and figures. Alzheimer's Association, (2012) . [ Links ]
3 Lyketsos C.G., Toone L., Tschanz J., Rabins P.V., Steinberg M., Onyike C.U. Population-based study of medical comorbidity in early dementia and cognitive impairment, no dementia (CIND): Association with functional and cognitive impairment: The Cache County Study. Am J Geriatr Psychiatry. 2005;13:656-64. [ Links ]
4 Barnes D.E., Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10:819-28. [ Links ]
5 Kuo T.C., Zhao Y., Weir S., Kramer M.S., Ash A.S. Implications of comorbidity on costs for patients with Alzheimer disease. Med Care. 2008;46:839-46. [ Links ]
6 Landi F., Gambassi G., Lapane K.L., Sgadari A., Gifford D., Mor V. Comorbidity and drug use in cognitively impaired elderly living in long-term care. Dement Geriatr Cogn Disord. 1998;9:347-56. [ Links ]
7 Sanderson M., Wang J., Davis D.R., Lane M.J., Cornman C.B., Fadden M.K. Co-morbidity associated with dementia. Am J Alzheimers Dis Other Dement. 2002;17:73-8. [ Links ]
8 Zekry D., Herrmann F.R., Grandjean R., Meynet M.-P., Michel J.-P., Gold G. Demented versus non-demented very old inpatients: The same comorbidities but poorer functional and nutritional status. Age Ageing. 2008;37:83-9. [ Links ]
9 Löppönen M.K., Isoaho R.E., Räihä I.J., Vahlberg T.J., Loikas S.M., Takala T.I. Undiagnosed diseases in patients with dementia: A potential target group for intervention. Dement Geriatr Cogn Disord. 2004;18:321-9. [ Links ]
10 Andersen F., Viitanen M., Halvorsen D., Straume B., Engstad T. Co-morbidity and drug treatment in Alzheimer's disease: A cross sectional study of participants in the Dementia Study in Northern Norway. BMC Geriatr. 2011;11:58. [ Links ]
11 Fick D., Kolanowski A., Waller J. High prevalence of central nervous system medications in community-dwelling older adults with dementia over a three-year period. Aging Ment Health. 2007;11:588-95. [ Links ]
12 Tjia J., Rothman M.R., Kiely D.K., Shaffer M.L., Holmes H.M., Sachs G.A. Daily medication use in nursing home residents with advanced dementia. J Am Geriatr Soc. 2010;58:880-8. [ Links ]
13 Fillit H., Hill J. Economics of dementia and pharmacoeconomics of dementia therapy. Am J Geriatr Pharmacother. 2005;3:39-49. [ Links ]
14 Psychiatric American Diagnostic and statistical manual of mental disorders: DSM–IV. 4th ed., American Psychiatric Association, (1994) .
15 Health World ICF Checklist for International Classification of Functioning, Disability and Health. [Internet]. World Health Organization, (2003) . [ Links ]
16 Guerreiro M., Silva A.P., Botelho M.A., Leitão O., Caldas A.C., Garcia C. Adaptação à população portuguesa do Mini Mental State Examination (MMSE). Rev Port Neurol. 1994;1:9-10. [ Links ]
17 Maroco J. Análise estatística com utilização do SPSS. Edições Sílabo, (2003) . [ Links ]
18 Kivipelto M., Ngandu T., Fratiglioni L., Viitanen M., Kareholt I., Winblad B. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556-60. [ Links ]
19 Xu W., Qiu C., Gatz M., Pedersen N.L., Johansson B., Fratiglioni L. Mid-and late life diabetes in relation to the risk of dementia. Diabetes. 2009;58:71-7. [ Links ]
20 Gorelick P.B., Scuteri A., Black S.E., DeCarli C., Greenberg S.M., Iadecola C. Vascular contributions to cognitive impairment and dementia. Stroke. 2011;42:2672-713. [ Links ]
21 Nunes B., Silva R., Cruz V., Roriz J., Pais J., Silva M. Prevalence and pattern of cognitive impairment in rural and urban populations from Northern Portugal. BMC Neurol. 2010;10:42. [ Links ]
22 Richard E., Ligthart S.A., van E.P.M., van W.A. Vascular risk factors and dementia: Towards prevention strategies. Neth J Med. 2010;68:284-90. [ Links ]
23 Marengoni A., Rizzuto D., Wang H., Winblad B., Fratiglioni L. Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc. 2009;57:225-30. [ Links ]
24 McCormick W.C., Kukull W.A., Belle G.V., Bowen J.D., Teri L., Larson E.B. Symptom patterns and comorbidity in the early stages of Alzheimer's disease. J Am Geriatr Soc. 1994;42:517-21. [ Links ]
25 Gure T.R., Kabeto M.U., Plassman B.L., Piette J.D., Langa K.M. Differences in functional impairment across subtypes of dementia. J Gerontol A Biol Sci Med Sci. 2010;65:434-41. [ Links ]
26 Szafara K.L., Kruse R.L., Mehr D.R., Ribbe M.W., van J.T. Mortality following nursing home–acquired lower respiratory infection: LRI severity, antibiotic treatment, and water intake. J Am Med Dir Assoc. 2012;13:376-83.
27 Schubert C.C., Boustani M., Callahan C.M., Perkins A.J., Carney C.P., Fox C. Comorbidity profile of dementia patients in primary care: Are they sicker. J Am Geriatr Soc. 2006;54:104-9. [ Links ]
28 Holmes H.M., Sachs G.A., Shega J.W., Hougham G.W., Cox D., Dale W. Integrating palliative medicine into the care of persons with advanced dementia: Identifying appropriate medication use. J Am Geriatr Soc. 2008;56:1306-11. [ Links ]
29 Hartikainen S., Lonnroos E., Louhivuori K. Medication as a risk factor for falls: Critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172-81. [ Links ]
30 Schneider L.S., Dagerman K., Insel P.S. Efficacy and adverse effects of atypical antipsychotics for dementia: Meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14:191-210. [ Links ]
31 Rocha V., Marques A., Figueiredo D., Pinto M., Sousa L. The (ab)use of antipsychotics in people with dementia according to the living conditions. 27th International Conference of Alzheimer's Disease International, London, UK, 7–12 March 2012 – Proceedings, Alzheimer's Disease International (ADI). Alzheimer's Society, 2012. pp. 107-11.
32 Rabins P.V., Blacker D., Rovner B.W., Rummans T., Schneider L.S. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer's disease and other dementias. Am J Psychiatry. 2007;164:(12 Suppl.)5-56. [ Links ]
33 Giron M.S., Wang H.X., Bernsten C., Thorslund M., Winblad B., Fastbom J. The appropriateness of drug use in an older nondemented and demented population. J Am Geriatr Soc. 2001;49:277-83. [ Links ]
34 Linjakumpu T., Hartikainen S., Klaukka T., Veijola J., Kivelä S., Isoaho R. Use of medications and polypharmacy are increasing among the elderly. J Clin Epidemiol. 2002;55:809-17. [ Links ]
*Autor para correspondência: Correio eletrónico: amarques@ua.pt
Received March 8, 2014 .Accepted July 30, 2014













