Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciência & Tecnologia dos Materiais
versão impressa ISSN 0870-8312
C.Tecn. Mat. v.21 n.3-4 Lisboa jul. 2009
Hydrogen, the ultimate clean fuel
César A.C. Sequeira
ICEMS-DEQB, Instituto Superior Técnico, TU Lisbon, Av. Rovisco Pais, 1049-001 Lisboa, Portugal. (cesarsequeira@ist.utl.pt; tel. 351-21-8417765)
ABSTRACT: Hydrogen is the ideal fuel from an ecological viewpoint. Here, brief considerations are presented on its generation, relevant properties, and use for combustion purposes.
Keywords: Hydrogen energy; Hydrogen fuel; Combustion.
RESUMO: O hidrogénio é o combustível ideal do ponto de vista ecológico. Este artigo apresenta breves considerações sobre a sua produção, propriedades mais relevantes e utilização como combustível.
Palavras chave: Energia do hidrogénio; Combustível de hidrogénio; Combustão.
1. INTRODUCTION
Hydrogen is the ideal fuel from an ecological viewpoint. It may, in principle, be derived from water using a non-fossil energy source (e.g. solar, geothermal, nuclear) and combust-ed back to water in a closed chemical cycle involving no release of carbonaceous pollutants. Hydrogen also has the potential to provide a storage component for renewable forms of energy and to transport this energy, via under-ground pipelines, from where it is produced to where it is needed. These topics are explored in some detail in a series of papers that I am preparing (e.g. Hydrogen Production, Hydrogen Distribution and Storage, Hydrogen End-Users, Fuel Cells to Generate Electricity) and will be published soon. Here, we are concerned with the combustion of hydrogen.
2. HYDROGEN GENERATION
Today, most hydrogen is produced by the steam reforming of natural gas, coal or naphtha, and by the partial oxidation of heavy oils to produce synthesis gas. Steam reforming of natural gas, by reacting methane with steam and air (or oxygen) over a nickel-based catalyst, i.e.
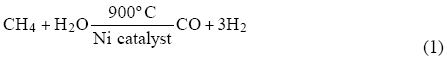
is the most efficient and widely used process and is combined with the water-gas shift reaction
CO + H2O ® CO2 + H2 (2)
to increase the amount of hydrogen produced. The final products are hydrogen and carbon dioxide. Steam reforming is very energy intensive since it operates at high temperatures (850-950 ºC) and high pressure (3.5 MPa). The thermal efficiency can reach 60-70 %. The catalyst is suspended in an array of tubes mounted in a hot box. Heat is provided by radiant transfer to the exterior of the tubes and is generated by the combustion of natural gas. The used reactor-heater system is large and bulky. Efforts are being made to reduce the size of the reformer and improve the efficiency of heat transfer. Obviously, the process is not environmentally benign due to large emissions of carbon dioxide. Typically, a steam reformer plant has a capacity of 104-105 t of hydrogen per year (i.e. 108-109 m3). In the USA alone, it is said that 90 billion cubic metres of hydrogen per year are produced by steam reforming of fossil fuels, predominantly methane, for use in the petrochemical and related industries. This is 50 % of the natural gas used in the USA.
In the alternative method for hydrogen production, i.e. the partial oxidation process, a fuel and oxygen (and sometimes steam) are combined in proportions such that the fuel is converted into a mixture of hydrogen and carbon monoxide. The amount of hydrogen is only about 75% of that produced by steam reforming. Depending on the composition of the feed and the type of the fossil fuel used, the partial oxidation process is carried out either catalytically or non-catalytical-ly. The latter approach operates at high temperatures (1100-1500 ºC) and can be applied to any possible feedstock, including heavy residual oils and coal. By contrast, the catalytic process is performed at a significant lower range of temperatures (600-900 ºC) and, in general, uses light hydrocarbon fuels as feedstock, e.g. natural gas and naphtha. The drawback to partial oxidation is that it requires the use of expensive oxygen (rather than air, which would dilute the product hydrogen with nitrogen).
Only a few percent of world hydrogen is produced by electrolysis and this is mostly as a by-product of the chlor-alkali process for the manufacture of chlorine and sodium hydroxide [1-3]. More speculative methods of hydrogen generation include: the thermolysis of water and hydrogen sulfide; the photochemical and photoelectrochemical de-composition of water; biological processes, e.g. solid biomass gasification, liquid biomass fermentation, algae photosynthesis, bacterial fermentation (see next paper on Hydrogen Production).
At current prices, hydrogen is used almost exclusively for the synthesis of ammonia, methanol, and other petro-chemicals – generally in a plant situated in the same petro-chemical complex as the reformer – and for petroleum refining (hydro-cracking, hydro-desulfurization, etc.). A small proportion of hydrogen is compressed into gas cylinders and sold for small-scale use in industrial plants and laboratories. Typical applications include the heat treatment of metals, the hydrogenation of oils to fats, the reduction of metallic oxides (as in the manufacture of refractory metals, nuclear fuels, etc.). With the growing importance of fuel cells, there will also be an increasing need for hydrogen as a fuel (see next paper on Fuel Cells to Generate Electricity). At present, however, the production of hydrogen as an all-purpose fuel is simply not economic, with so many cheap fossil fuels available.
3. TECHNICAL PROPERTIES
The relevant technical properties of hydrogen as a fuel, in comparison with those of conventional and synthetic fuels are listed in Table 1. The most characteristic features of hydrogen are its low boiling point and its exceptionally low density in both the gas and the liquid state. By virtue of this latter feature, hydrogen has an extremely high heating value on a unit mass basis. On the other hand, the heating value of liquid hydrogen per unit volume is less than that of other liquid fuels.
Table 1. Technical comparison of hydrogen with other fuels.
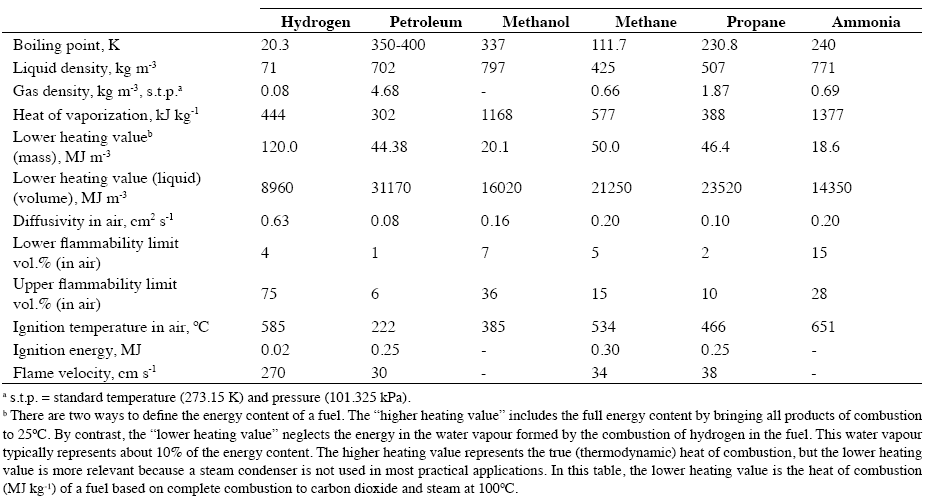
The properties listed in the lower half of Table 1 are relevant to safety considerations. Hydrogen is notable for its very rapid diffusivity in air, its high value for the upper flammability limit in air, its exceptionally low ignition energy, and its remarkably high flame velocity. This combination of properties makes it a unique fuel from both utilization and safety aspects. Hydrogen has a poor safety reputation, largely on account of the Hindenburg airship disaster in 1937. It has recently been shown, however, that the Hindenburg fire had more to do with the flammability of the fabric chosen for the airships envelope than with the fact that it was filled with hydrogen. Nevertheless, airships and balloons are now filled with helium and not hydrogen. There is, however, considerable industrial experience of the safe handling of hydrogen gas in bulk, both in refineries and in chemical plants. The general opinion of those involved in such operations is that hydrogen is safe provided that its properties are clearly understood and well-defined safety regulations are followed. A positive safety feature of hydrogen is that in the event of a fire, the low luminosity of the flame restricts the emission of thermal radiation to less than one-tenth of that from hydrocarbon flames. Thus, bystanders are much less likely to suffer radiation burns.
The low density and high diffusivity of hydrogen results in a very rapid dispersal of liquid hydrogen after a spillage, so that the risk of fire persists for a much shorter period than with other liquid fuels. All confined spaces in which hydrogen is handled must be well ventilated. The low ignition energy makes it necessary to exclude all sources of sparks, such as electric motors, synthetic garments, and steel tools. When these and similar precautions are observed, experience with handling hydrogen in bulk has been favourable.
The combustion characteristics of hydrogen are quite different from those of natural gas or LPG (liquefied petroleum gas) and this requires a modified burner design. The principles involved are well understood and the design of a burner for pure hydrogen presents no serious difficulties. The low ignition energy of hydrogen favours the use of catalytic burners that are of higher efficiency and lower flame temperature than conventional burners.
4. HYDROGEN AS A FUEL
The use of hydrogen as a fuel for internal combustion engines was first demonstrated in the 1930s. From the 1970s to the present day, there has been a continuing and strong interest in hydrogen energy and numerous research engines tend to exhibit pre-ignition, back-fire, and knock. These phenomena are caused by the low ignition energy and high flame speed of hydrogen. These problems have now been largely overcome. By using lean mixtures of hydrogen in air, it is possible to reduce NOx emissions to well below that of a conventional petrol engine. In principle, therefore, the practical realization of pollution-free, hydrogen fuelled engines appears to present no major difficulties.
Several car companies are experimenting models fitted with hydrogen-fuelled engines. Ford has demonstrated a mid-size saloon (Mondeo) with a modified 2-litre petrol engine. The hydrogen fuel is stored under pressure (about 30 MPa) in cylinders that are located in the boot (trunk). Meanwhile, BMW has equipped several of its large (7-series) cars with dual-fuel capacity, viz, hydrogen and petrol. In this case, liquid hydrogen is used and is stored in cryogenic tanks of 120-litre capacity, again located in the boot.
The problem of how to store hydrogen for use with internal-combustion engines is formidable. Pressurized gas cylinders are heavy and bulky to accommodate, but recently lightweight gas cylinders based on carbon-fibre composites are capable of storing hydrogen at over 30 MPa pressure have been developed. Liquid hydrogen is expensive to produce, both in monetary terms and also in terms of the energy lost in the liquefaction process (see next paper on Hydrogen Distribution and Storage). The electricity consumed in the liquefaction of hydrogen depends on the size of the plant and ranges from 10 to 20 kWh per kg of hydrogen. Also, the cryogenic storage tanks and associated equipment are costly and take up considerable space. A third option, that of storage as a metal hydride, is discussed in some of my papers [4-8]. Finally, as far as road vehicles are concerned, there is the problem of providing a network of refuelling stations at which either high-pressure hydrogen or liquid hydrogen can be supplied. This will be a much more difficult task in their current exercise of providing a distribution infrastructure for LPG.
Some interest has been expressed in the possibility of producing a new passenger aircraft, possibly supersonic, fuelled by hydrogen. Liquid hydrogen fuel is attractive to aircraft designers on account of its low mass, which would allow either increased range or payload. On the other hand, there is a space problem. In rounded terms, the data given in Table 2 show that liquid hydrogen occupies four times the volume of hydrocarbon fuels (i.e. kerosene, the conventional aircraft fuel), but has little more than one-third of the mass. A major design problem is, therefore, where to accommodate the liquid hydrogen in the aircraft. Possibilities include the use of tanks (pods) suspended below the wings (taking advantage of the low mass), or storage above or even within the fuselage. There are also operational problems, mainly the logistics, costs and safety of supplying liquid hydrogen in the required quantities at major airports. These ideas were first discussed in the early 1970s. Later, Lockheed Corporation proposed to the US government that two of the Companys Tristar freighters should be converted to liquid hydrogen (LH2) and operated across the Atlantic as a demonstration project.
Table 2. Mass and volume comparisons of kerosene and liquid hydrogen (lower heating values).
|
| Mass | Volume | ||
| (kg per GJ) | Indexa | (m3 per GJ) | Indexa | |
| Liquid hydrogen | 8.33 | 0.38 | 0.112 | 4.00 |
| Kerosene | 22.0 | 1.0 | 0.28 | 1.0 |
a Taking mass and volume for kerosene as unity.
Nothing came of this and the idea was abandoned, as was another proposal in 1980 for An International Research and Development Programme on LH2-fuelled Aircraft. At present, there is little prospect of this activity being revived, thanks to the availability and low cost of conventional (fossil) fuels, although it is pertinent to point out that there is some interest in a new generation of supersonic aircraft, and also that there is general concern over the release of greenhouse gases in the stratosphere by supersonic aircraft.
To assist the reader, a brief summary of synthetic fuels that have been, or are being, used is given in Figure 1. Further considerations on hydrogen energy are given in references [9] to [15].
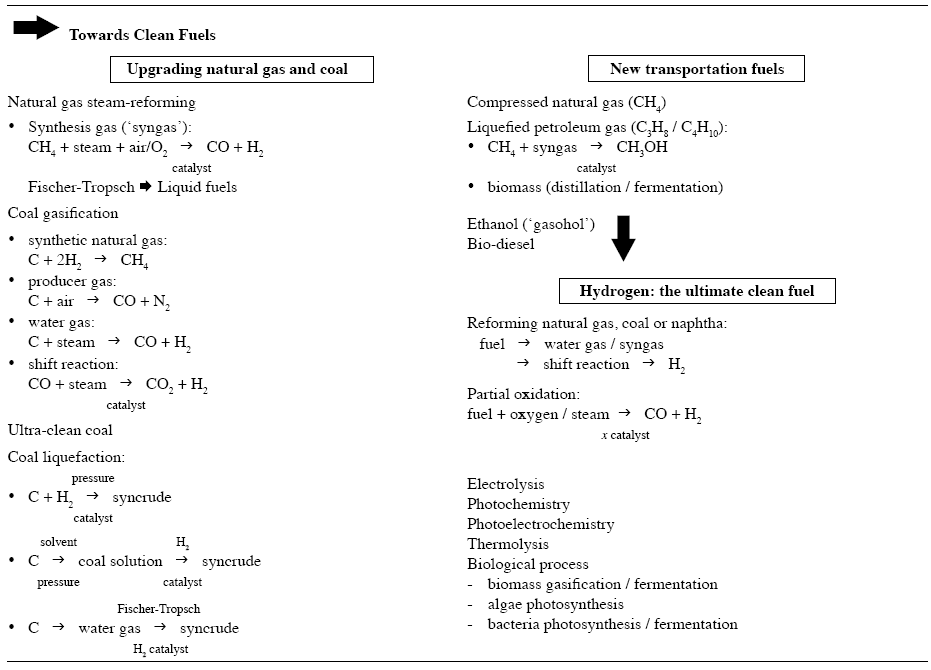
Fig. 1. Summary of synthetic fuels.
REFERENCES
[1] C. A. C. Sequeira and D. M. F. Santos, J. Braz. Chem. Soc. 20 (3) (2009) 387.
[2] C. A. C. Sequeira and D. M. F. Santos, in Energy Conversion: New Research, Wenghong Lin (Ed.), Nova Science Publishers, New York, p. 31-87 (2008).
[3] C. A. C. Sequeira, C. Tecn. Mat. 21 (1/2) (2009) 85. [ Links ]
[4] C. A. C. Sequeira, Y. Chen and D. M. F. Santos, J. Electrochem. Soc. 153 (10) (2006) A1863.
[5] Y. Chen, C. A. C. Sequeira, X. Song, C. Chen, Z. Phys. Chem. 220 (2006) 631.
[6] Y. Chen, X. Wang, L. Chen, C. Chen, Q. Wang and C. A. C. Sequeira, J.Alloys Comp. 421 (2006) 223.
[7] Y. Chen, C. A. C. Sequeira, C. Chen, X. Wang and Q. Wang, Int. J. Hydrogen Energy 28 (3) (2003) 329.
[8] Y. Chen, C. A. C. Sequeira, C. Chen and Q. Wang, J. Alloys Comp. 354 (2003) 120.
[9] C. A. C. Sequeira, P. S. D. Brito, A. F. Mota, J. L. Carvalho, L. F. F. T. T. G. Rodrigues, D. M. F. Santos, D. B. Barrio and D. M. Justo, Energy Conv. & Manag.48 (7) (2007) 2203.
[10] C. A. C. Sequeira, in Annualia, Editorial Verbo, Lisboa, p. 155-177 (2005/2006).
[11] C.-J. Winter and J. Nitsch (Eds.), Hydrogen as an Energy Carrier, Springer-Verlag, Berlin, (1988).
[12] H. Wendt (Ed.), Electrochemical Hydrogen Technolo-gies, Elsevier Science Publishers B.V., Amsterdam, (1990).
[13] T. Ponce de Leão, Ingenium, II Série, 112 (2009) 28.
[14] Key World Energy Statistics from the IEA, 2003 Edn., International Energy Agency, Paris, ( 2003).
[15] M. A. Weiss, J. B. Heywood, E. M. Drake, A. Schafer and F. F. An Yeung, Energy Laboratory Report MIT EL 00-003, Massachusetts Institute of Technology, Cambridge, MA, (2000).













