Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciência & Tecnologia dos Materiais
versão impressa ISSN 0870-8312
C.Tecn. Mat. v.21 n.3-4 Lisboa jul. 2009
Coated rubber granulates obtained from used tyres for use in sport facilities: A toxicological assessment
H. I Mota1, J.F.P.Gomes1,2*, J.C.M Bordado1, M.M.C Pereira3, G.M.S. Felisberto 3, A. Ribeiro 3,
V. M. Pampulim4, I. Veloso4, M.L.B. Custódio4
1IBB/Centre for Chemical and Biological Engineering, Instituto Superior Técnico,
Av. Rovisco Pais, 1049-001 Lisboa, Portugal
2Chemical Engineering Department, Instituto Superior de Engenharia de Lisboa,
R. Conselheiro Emídio Navarro, 1, 1959-007 Lisboa, Portugal
3LAIST, Instituto Superior Técnico, Av. Rovisco Pais, 1049-001 Lisboa, Portugal
4Recipneu, Parque Industrial de Sines, Apt. 26, 7521-901 Sines, Portugal
ABSTRACT
Reuse of tire crumb in sport facilities is currently a very cost-effective waste management measure. Considering that the incorporation of the waste materials in artificial turf would be facilitated if the rubber materials are already coloured of green, coatings were developed for this purpose. This study presents the toxicological and environmental assessment made comparing the obtained emissions to the environment in terms of PAHs, heavy metals and Ecotoxicity for coated and non-coated rubber granulates. It was concluded that, among two types of coatings tested, one is particularly effective in reducing emissions to the environment meeting, at the same time, the requirements of adherence and colour stability.
KEYWORDS
Tire crumb; Coated rubber granulate; Artificial Turf; Toxicological Assessment
RESUMO
A utilização de granulado técnico de borracha resultante da reciclagem de pneus é, actualmente, uma medida eficiente de gestão de resíduos. Com vista ao aumento da possibilidade de utilizações para este granulado procedeu-se à sua coloração artificial com pigmentos verdes que facilitam a sua incorporação em relva artifical para uso em recintos desportivos, Este estudo refere-se à determinação das características de toxicidade dos materiais obtidos, comparando-se as emissões de PAHs, metais pesados e ecotoxicidade para os grânulos coloridos e grânulos tal-qual. Concluiu-se que os grânulos coloridos têm menores emissões para o ambiente, cumprindo simultaneamente com os requisitos de aderência e estabilidade de cor.
1. INTRODUCTION
Synthetic turf areas are, nowadays, well established in almost all sport facilities. Many synthetic turf fields consist not only of artificial grass but also rubber granulated material that is used as infill. In fact, disposal of used tires has been, since long, a major problem in solid waste management [1]. Thus, recovery and recycling of rubber from used tires is an important environmental protection measures, which lead to the development of processes capable of using the rubber contained in the tires. Several of these processes involve the conversion of the tire into more manageable physical materials, such as the manufacture of tire crumbs, or rubber granulates, from spent vehicle tires.
Most available processes of this nature comprise the production of rubber granulates by mechanical shredding of tires, which could also be achieved by cryogenic means. The obtained rubber granules exhibit certain differences regarding the original raw materials and its composition largely depends on the type and condition of the used tires [2].The shape and dimensions of the rubber granules is mainly dependant of the type of shredding process used (mechanical or cryogenic).
Recipneu, located in Sines, Portugal, is currently using the cryogenic process for producing vulcanized rubber granulates. This process, depicted in Figure 1, involves an extreme cooling of the roughly shredded tires using liquid nitrogen at -196 ºC, followed by the action of high impact forces, resulting in obtaining a series of rubber granulates, with different sizes, with the shape of small cubes having smooth surfaces, almost non-porous. The whole process takes place in an inert atmosphere during a very short time.
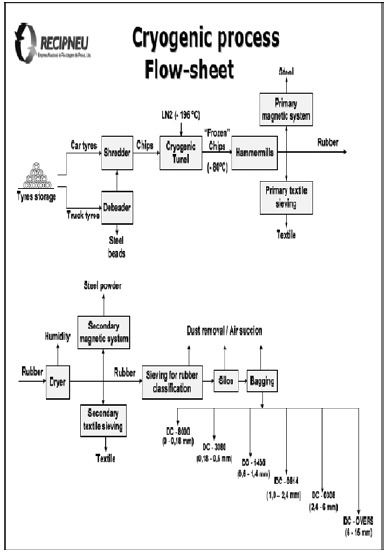
Figure 1. Process flow sheet for production of rubber granulate by cryogenic route
The cryogenic process does neither chemically or thermically degrades the molecular chains of the rubber polymers or its vulcanization condition. The cryogenically obtained rubber granulate thus presents several advantages over the mechanically obtained ones as they do not suffer thermal degradation, and their behaviour is highly elastic [2].
Apart from that, they do not have any particular odour which recommends its use for pavements and artificial grass in indoor sports facilities. Also, sport surfaces made from waste tires have the potential to reduce users injury due to falls. In fact, the demand for these rubber granulates concerning its use for artificial turf has been increasing recently, which can be attributed to characteristics such as the regularity of particles and its specific area, resulting in an excellent drainage of water and less superficial wear. Rubber granulates obtained cryogenically can have a size distribution that can be adjusted to the required subsequent utilization: usually the fraction smaller than 0.6 mm is more adequate for incorporation in bitumen used for road and pavement construction, whereas the fraction ranging from 0.6 to 2.5 mm is more adequate to be used as filling material in synthetic turf (based on polyethylene and polypropylene).
Figure 2, shows the typical size distribution of particles for the rubber granulate (Lot Reference DC-0814) obtained cryogenically as analyzed by an Ankersmid Eyetech Particle Size and Shape Analyzer. This histogram clearly shows that the average nominal diameter ranges from 1.5 to 2.2 mm. The actual specifications recommend a size range of 1.5 to 2.4 mm for incorporation in artificial turf.
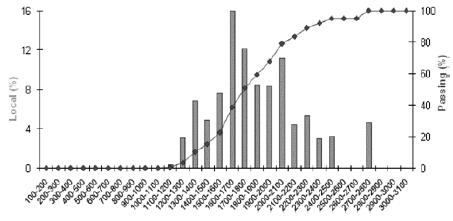
Figure 2. Typical size distribution of particles determined for rubber granulate obtained cryogenically (Lot Reference DC-0814)
Figure 3 shows the typical morphology of the rubber granulate particles, also obtained with the Ankersmid Eyetech analyzer.
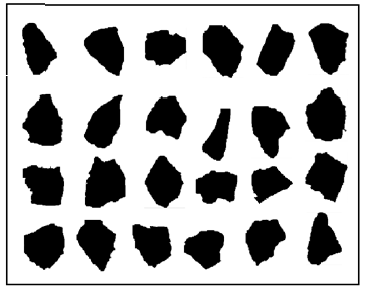
Figure 3. Representation of the typical shape of rubber granulate obtained cryogenically
(Lot Reference DC-0814)
2. UTILIZATION OF RUBBER GRANULATES IN ARTIFICIAL TURF
In order to increase the utilization possibilities of the rubber granulates produced by Recipneu, studies were made aiming to obtain synthetic coatings for these granulates, in a colour (green) that would facilitate its incorporation in artificial turf to be used for sports facilities.
The main requirements were to obtain a coating that would adhere efficiently to the rubber granules, maintaining its original elastic properties, colour stability, even when subjected to adverse weather conditions, resistance to wear and also temperature. It was also expected that this coating will be effective in reducing emissions of leachates, and airborne substances, thus being beneficial both in terms of the health of exposed individuals and also to the environment as a whole.
Complying with these requirements, two efficient coatings were obtained, one based on emulsified PVC (poly-vinyl-chloride), referenced as DC-0814/R1 and another one based on a reticulated alquidic polymer, referenced as DC-0814/R2. Both coating formulations comprise colour additives and also a flame-retardant agent.
As recycled tires may contain several substances of high concern [3], such as Polycyclic Aromatic Hydrocarbons (PAHs) and heavy metal species; the environmental and health compatibility of the synthetic turf has to be guaranteed during its use. Environmental and health risks of loading in sports areas with used tires might be the possible release of dangerous particles to the air, contamination of soil and groundwater by soluble pollutants of the materials extracted by rain water and the health hazard for residents and users of sport areas by inhalation of pollutants [4].
This has led to the development of previous studies of environmental assessment concerning certain classes of pollutants, such as heavy metals [3], [5], [6], [7], inorganic species [8], from tire leachates [9], [10], as well as toxicity assessments using biological organisms [11]-[13]. However, full assessments for all involved potentially resulting pollutants have not been systematically performed so far.
This study aimed to perform a more complete evaluation of the major potential critical factors related with the release of pollutants from coated rubber granulates by comparison with uncoated ones.
2. EXPERIMENTAL
Next section describes the toxicity assessment tests made comparing raw rubber granulates and coated rubber granulates (formulations R1 and R2) obtained from the same raw materials.
2.1. Inhalable dust
The percentage of inhalable dust was measured using the laser channel of the Ankersmid Eyetech analyzer, using a specific lens capable of measuring particles larger than 0.1 µm in diameter. The performed analysis made compared samples of rubber granulate obtained cryogenically and also samples of rubber granulate obtained mechanically, by another producer, as well as granulate samples resulting from a semi-cryogenic process. The obtained results are presented in table 1.
Table 1. Measured percentage of inhalable particulate in rubber granulate samples
|
| Inhalable particulate (%) | |||
| Sample | Cryogenic DC-1430 | Cryogenic DC-0102 | Semi-Cryogenic RA-1435 | Mechanical Fr_1 |
| PM2,5 | 0.34 | 0 | 0.48 | 16.24 |
| PM10 | 1.95 | 9.14 | 3.96 | 35.17 |
These tests show that both cryogenic and semi-cryogenic granulates intrinsic content in inhalable particulate is somewhat lower than the content of analyzed mechanical granulate. Thus, it can be expected that artificial turf incorporating cryogenic or semi-cryogenic rubber granulates will result in lower airborne inhalable particulate emissions, when compared with artificial turf incorporating mechanical rubber granulates.
2.2. PAHs
PAH content was measured both in the rubber granulates and also in resulting water leachates. PAH determination in rubber granulates was performed according to the international standard ISO 18287 [14] a first extraction is made with acetone and later on with petroleum ether. As the material has high organic matter content, the extract was passed through a silica gel column and the resulting sample was then concentrated in cyclohexane before being injected into a gas chromatograph equipped with a mass spectrometry detector (GC-MS) equipment Thermo Scientific GC Ultra. The obtained results are presented in Table 2.
Table 2. PAH content in raw and coated rubber granulate samples, Lot DC-0814
| PAH content (mg/Kg) | DC‑0814 | DC-0814/R1 | DC-0814/R2 |
| Naphthalene | 0.16 | 0.13 | 0.35 |
| Acenaftalene | 0.27 | 0.27 | 0.38 |
| Acenaftene | 0.04 | <0.08 | <0.08 |
| Fluorene | 0.12 | 0.13 | 0.18 |
| Phenantrene | 1.41 | 1.23 | 1.58 |
| Anthracene | 0.13 | 0.13 | 0.19 |
| Fluoranthene | 4.50 | 3.74 | 5.98 |
| Pyrene | 14.42 | 13.95 | 21.10 |
| Benzo(a)anthracene | 1.31 | 0.92 | 0.82 |
| Chrysene | 2.83 | 2.12 | 2.70 |
| Benzo(b)fluoranthene | <0.08 | <0.08 | <0.08 |
| Benzo(k)fluoranthene | <0.08 | <0.08 | <0.08 |
| Benzo(a)pyrene | 1.19 | <0.08 | 0.43 |
| Indene(1,2,3)-cd-pyrene | <0.08 | <0.08 | <0.08 |
| Dibenzo(a,h)anthracene | <0.08 | <0.08 | <0.08 |
| Benzo(ghi)perylene | <0.08 | <0.08 | <0.08 |
| SUM | 26.77 | 23.15 | 34.17 |
Water leachates, both from raw and coated rubber granulates, were prepared according to the German standard DIN 38414-4 [15], using two leaching cycles: the first leachate was collected at 24 h, and the obtained product was leached again for more 24 h, thus accounting for the 48 h prescribed by the German standard DIN V 18035-7 [16]. Leachates were then filtered at 0.45 µm, and extracted with dichloromethane, concentrated and cleaned using a silica gel column, concentrated again in cyclohexane and analyzed in the GC-MS equipment referenced before. The obtained results are presented in Table 3.
Table 3. PAH content in water leachates prepared from raw and coated rubber granulate samples, Lot DC-0814
| PAH content (mg/Kg) | DC‑0814 | DC-0814/R1 | DC-0814/R2 |
| Naphthalene | <0.005 | <0.001 | <0.001 |
| Acenaftalene | <0.003 | <0.001 | <0.001 |
| Acenaftene | <0.003 | <0.001 | <0.001 |
| Fluorene | <0.003 | <0.001 | <0.001 |
| Phenantrene | <0.003 | <0.001 | <0.001 |
| Anthracene | <0.003 | <0.001 | <0.001 |
| Fluoranthene | <0.003 | <0.001 | <0.001 |
| Pyrene | <0.003 | 0.001 | <0.001 |
| Benzo(a)anthracene | <0.003 | <0.001 | <0.001 |
| Chrysene | <0.003 | <0.001 | <0.001 |
| Benzo(b)fluoranthene | <0.003 | <0.001 | <0.001 |
| Benzo(k)fluoranthene | <0.003 | <0.001 | <0.001 |
| Benzo(a)pyrene | <0.003 | <0.001 | <0.001 |
| Indene(1,2,3)-cd-pyrene | <0.003 | <0.001 | <0.001 |
| Dibenzo(a,h)anthracene | <0.003 | <0.001 | <0.001 |
| Benzo(ghi)perylene | <0.003 | <0.001 | <0.001 |
| SUM | <0.050 | 0.016 | <0.016 |
2.3. Heavy metals
This study also comprised the analysis of significant heavy metals content in acidic leachates. The determined heavy metal species were the ones that previous studies [3], [7] showed to be the ones of more concern due to its high content in tires, and also that are particularly harmful to the environment and, therefore, are subjected to specific limits in regulations such as the German standard DIN 18035-7 [16]: cadmium (Cd), chromium (Cr), mercury (Hg), lead (Pb), tin (Sn) and zinc (Zn). It should be noted that hexavalent chromium (Cr6+) was not determined as the measured values for total chromium were lower than the prescribed limit value for hexavalent chromium.
Leaching tests in acidic medium were performed in accordance with the German standard DIN 18035-7 [16], which proved to be adequate for environmental contamination assessments [17]. Both raw and coated rubber granulate materials were mixed with water in a weight proportion 1:10. The mixture was saturated with carbon dioxide bubbling at a flow rate of 50 mL/min, resulting in an acidic pH (around 4 to 5) during leaching tests. The first cycle lasted for 24 h, and then the mixture was filtered at 0.45 µm resulting in the 24 h leachates. The solid material was subject to another leaching test, using the same conditions, for more 24 h, resulting in the 48 h leachates.
The heavy metals content was determined by ICP/OES using Perkin Elmer Optima 3000 equipment, using a coupled Perkin Elmer graphite chamber, for all metals except mercury, where a cold vapour device Perkin Elmer FIMS 400 was used. The obtained results are presented in table 4.
Table 4. Heavy metals content in acidic water leachates prepared from raw and coated rubber granulate samples, Lot DC-0814
|
| DC‑0814 | DC-0814/R1 | DC-0814/R2 | Limits | |||
| Leaching time | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 48 h |
| Cd (mg/L) | 0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.005 |
| Cr (mg/L) | 0.003 | 0.002 | <0.001 | <0.001 | 0.002 | <0.001 | <0.05 |
| Hg (mg/L) | <0.0008 | <0.0008 | <0.0008 | <0.0008 | <0.0008 | <0.0008 | <0.001 |
| Pb (mg/L) | 0.003 | 0.006 | 0.003 | <0.003 | 0.019 | 0.006 | <0.04 |
| Sn (mg/L) | <0.005 | <0.005 | 0.02 | 0.009 | 0.47 | 0.31 | <0.05 |
| Zn (mg/L) | 6.9 | 1.6 | 1.7 | 0.3 | 3 | 0.9 | <3 |
2.4. Ecotoxicity
Toxicity tests were performed using luminescent bacteria Vibrio Fischeri which has been previously employed in environmental toxicity tests for this type of materials [11], provides much more fast results than toxicity tests done with the microcrustacean Daphnia Magna or algae and this test is considered as reliable for Ecotoxicity assessment [18].
Results presented on Table 5 are given as derived toxic units (TU). This calculated value is derived from a probit analysis to determine the estimated concentration that produces a noticeable effect in 50% of the organisms tested (EC50), which may be a lethal effect (LC50) or an inhibitory effect (IC50). The level so derived is inverted and multiplied by 100, being expressed as a percentage.
Table 5. Toxicity (EC50) of raw and coated rubber granulate samples, Lot DC-0814
|
| DC‑0814 | DC-0814/R1 | DC-0814/R2 |
| Toxicity (EC50) | 68% | 56% | 34% |
3. DISCUSSION
It should be noted, again, that the developed coating material and procedure aimed not only to achieve a stable coloured coating adhering efficiently to the rubber granulate, but also a coating that would be effective in reducing emissions of leacheates and airborne substances. The first objective was achieved with both tested formulations for the coatings: R1 and R2. However, certain differences were found between coatings R1 and R2, regarding the second objective. In fact, considering PAH content in rubber granulate samples, it can easily be noted, from Table 2, that coating R2 has higher contents of certain PAHs species when compared to the raw, non-coated rubber granulate of the same Lot. This is particularly evident for naphthalene, acenaftalene, fluorene, fenantrene, anthracene, fluoranthene and pyrene. This could be due to the chemical composition of the coating, consisting, in this case of a alquidic polymer. Nevertheless, and is spite of this higher PAHs content, it should be noted that water leaching of this coated R2 rubber granulates, still results in very low leaching of these toxic compounds, as shown in Table 3, where the R2 coating exhibits the same behaviour as R1 coating regarding PAHs leaching. In fact, the determination of individual PAH species led to values below the detection limit, the sum for all PAH species being possibly lower for coated materials, R1 and R2, than for the raw non-coated material. It should be noted, however, that, bearing in mind the obtained levels one can conclude that PAH leaching is negligenciable.
In what concerns heavy metals, the majority of determined concentration in the leachates is very small, which points out to the fact that coatings are effective in preventing leaching of pollutants. The only exception seems to occur in what regards tin, which is particularly noticeable for coating R2. This is certainly due to the use of tin catalysts that are usually used for obtaining alquidic resins [19].
However, it should be noted that the R1 coated rubber granulate shows, as seen in Table 4, heavy metals content in the acidic water leachates considerably lower than the limit values imposed by DIN 18035-7 for all metals at 48 h leaching. For R2 coated rubber granulate the only exception is for tin, where the obtained value of 0,31 mg/L at 48 h leaching surpasses the limit value of 0.05 mg/L.
Regarding ecotoxicity tests, Table 5 shows that both coatings, R1 and R2, show a lower toxicity when compared with the non-coated rubber granulates.
4. CONCLUSIONS
The study presented in this paper points out that the R1 coating, based on emulsified PVC, is not only fulfilling the requirements of adherence and colour stability desired for this particular application but is also effective in reducing environmental emissions from the rubber granulate materials, namely in terms of leaching of pollutants such as PAHs and heavy metals. The other coating tested, R2, based on a reticulated alquidic polymer, shows also good characteristics but has a leaching value for tin that surpasses the regulated limit. However, due to its good adherence and stability characteristics, we intend to investigate further the leaching behaviour of this type of coatings for rubber granulates, using alquidic resins not so contaminated with traces of tin from production catalysts.
ACKWOLEDGMENTS
Financial support by AdI - Agência de Inovação, through contract IDEIA 70/00354 is gratefully acknowledged.
References
[1] U.S. Environmental Protection Agency. Manufacturing from Recyclables: 24 Case Studies of Successful Recycling Enterprises; EPA 530R95001; US Government Printing Office, Washington DC, 2000
[2] M. Wilczek, J. Berthig, D. Hintermann, Optimised technology for cryogenic production, Int. J. Mech. Proc., 74 (2004) 5425-5434 [ Links ]
[3] B. Bocca, G. Forte, F. Petrucci, S. Costantini, P. Izzo, Metals contained and leached from rubber granulates used in synthetic turf areas, Sci. Tot. Env., 407-7 (2009), 2183-2190
[4] Swedish Chemicals Inspectorate, Kemi, Synthetic turf from a chemical perspective – a status report, Sundbyberg, Sweden, 2006
[5] J. Homer, Environmental health implications of heavy metal pollution from car tires, Rev. Env. Health, 11 (2006), 175-178
[6] T. Councell, K. Duckenfiled, E. Landa, E. Callender, Tire-wear particles as a source of zinc to the environment, Env. Sci. Tech., 38 (2004), 4206-4214
[7] K. Adachi, Y. Tainosho, Characterization of heavy metal particles embedded in tire dust, Env. Int., 30 (2004), 1009-1017
[8] G. San Miguel, G., Fowler, C. Sollars, The leaching of inorganic species from activated carbons produced from waste tire rubber, Wat. Res., 36 (2002), 1939-1946
[9] M. Gualteri, M. Andrioletti, C. Vismara, M. Milani, M. Camatini, Toxicity of tire debris leachates, Env. Int., 31 (2005), 723-730
[10] S. Nelson, G. Mueller, D. Hemphill, Identification of tire leachate toxicants and a risk assessment of water quality effects using tire reefs and canals, Bull. Env. Cont. Tox., 52 (1994), 574-581
[11] D. Birkholz, K. Belton, T. Guidotti, Toxicological evaluation for the hazard assessment of tire crumb for use in public playgrounds, J. Air Waste Man. Assoc., 53 (2003), 903-907
[12] K. Day, K. Holtze, S. Metcalfe, C. Bishop, B. Dukta, Toxicity of leachate from automobile tires to aquatic biota, Chemos., 27 (1993), 665-675
[13] A. Wik, G. Daves, Acute toxicity of leachates of tire wear material to Daphnia magna – variability and toxic components, Chemos., 64 (2006), 1777-1784
[14] ISO 18287 - Soil quality. Determination of polycyclic aromatic hydrocarbons (PAH). Gas chromatographic method with mass spectrometric detection (GC-MS), International Standards Organization, Geneva, 2006
[15] DIN 38414-7. German standard methods for the examination of water, waste water and sludge; sludge and sediments; determination of leachability by water, Deutsches Institut für Normung e. V., Berlin, 1984
[16] DIN V 18035-7 - Sports grounds. Part 7: Synthetic turf areas, Deutsches Institut für Normung e. V., Berlin, 2002
[17] J. Gomes, C. Pinto, Leaching of heavy metals from steelmaking slags, Rev. Metal. Mad., 42 (2006), 409-416
[18] Biological Test Method: Toxicity Test Using Luminescent Bacteria; EPS 1/RM/24; Environment Canada, Ottawa, Ontario, 1992
[19] Campbell, I., Introduction to Synthetic Polymers, Oxford University Press, 2nd Ed., Oxford, 2000













