Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Silva Lusitana
versão impressa ISSN 0870-6352
Silva Lus. vol.19 n.Especial Lisboa 2011
The Insecticides Obtained from Turpentine
Miguel Pestana
INRB, IP – Instituto Nacional dos Recursos Biológicos. Quinta do Marquês, Av. da República, 2780-159 OEIRAS
Abstract
Over a period of millions of years there has been a close association, evolution and adaptation between plants and insects. The plants have developed terpenes and their derivatives for their own protection and pollination. The insects interact with these substances, ingesting and using them or their biosynthesized derivatives for their own bio-regulation. They have used them as sex hormones, growth and development hormones and pheromones of behaviour such as feeding, aggregation, alarm, and defence. It is also known that turpentine (the volatile fraction of the pine resin, obtained by its distillation) and some of its derivatives have in their constitution substances known as insecticides. This article reviews the most important products - the thiocyanates, the chlorinated terpenes, the chrysanthemum-carboxylic acids, the terpenes and derivatives and the terpene polymers - discussing their main characteristics, chemical synthesis, advantages and disadvantages.
Key words: Insecticides; turpentine; terpenes; toxicology; production
Os Inseticidas Obtidos de Terebentina
Sumário
Ao longo de milhões de anos tem existido uma associação íntima e uma adaptação e co-evolução entre plantas e insetos. As plantas desenvolveram terpenos e derivados para a sua proteção e polinização. Os insetos interagem com estas substâncias, ingerindo-as ou os seus derivados e usando-as na sua própria bioregulação. Tais substâncias podem ser usadas como hormonas sexuais, de desenvolvimento e crescimento, ou como feromonas de comportamento, tais como alimentação, agregação, alarme e defesa. Sabe-se hoje que o terpeno (fração volátil da resina do Pinheiro obtida por destilação) e alguns dos seus derivados possuem na sua constituição substâncias com efeito inseticida. Neste artigo revêm-se os produtos mais importantes, tais como tiocianatos, terpenos clorados, ácidos crisantemumcarboxílicos, terpenos e derivados e polímeros de terpenos, discutindo as suas características principais, síntese química, vantagens e desvantagens.
Palavras-chave: Inseticidas; aguarrás; terpenos; toxicologia; produção
Introduction
Natural products have been used as insecticidal agents since antiquity. In addition to sulphur, red squill, hellebore, arsenic and mineral oil, turpentine was recommended for repelling and killing insects.
Insect control became more predictable with the introduction and use of pyrethrum in the USA during the 1880s. Because of its high cost, additives to increase or enhance its activity were eagerly sought. Pine oil was found to be effective. Later the ethylene glycol ether of a-pinene (a-terpinyl b-hydroxyethyl ether) was found to enhance even more the activity of sprays containing pyrethrum and rotenone (HUMPHREY, 1938).
Considerable experimental work was done with other terpene alcohols, ethers and esters to determine their possibilities as activators and as insecticides (PIERPONT, 1939 and 1941). Terpin diacetate and dipropionate (BORGLIN, 1941) and other esters and ethers of terpene alcohols, such as terpineol, and fenchyl and bornyl alcohols (Figure 1), all had some activating proprieties (ENAN, 2001; HEBEISH, 2008). Further development led to the introduction of halogen into terpene esters – e.g., fenchyl chloroacetate. These, while not insecticidal, were also activators for pyrethrum. Finally, replacement of the halogen with the thiocyanate group gave terpene esters of outstanding insecticidal activity.
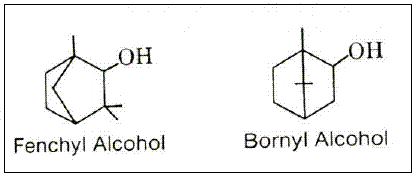
Figure 1 – Fenchyl and Bornyl Alcohol
The thiocyanates
The isobornyl thiocyanoacetate was introduced in 1940 by Hercules Powder Co. (BORGLIN, 1940 and 1940a) as an insecticide for the control of household and livestock pests. The development of this product was made during the war years of the 1940s when imported pyrethrum was in short supply. Thanite insecticide was first made from secondary terpene alcohol fractions (fenchyl and bornyl alcohols), but later commercial production was from camphene, obtained by isomerisation of a-pinene. In application it has some similarity to pyrethrum, causing quick knockdown or paralysis, high insect kill and low mammalian toxicity (HERCULES, 1954).
It is a product that gives no skin reaction on humans and does not behave as a sensitizer, as a primary irritant. It may be considered safe in a proper vehicle at lower use concentrations of 3 to 5%. Eye exposure causes primary irritation with rapid recovery upon removal from exposure.
Chlorinated terpenes
In the 1940s, Hercules Powder Co. had developed the synthesis of insecticides from terpenes. This work was a result of the disclosure in 1943 of the German Army’s use of DDT in North Africa. Chlorinated terpene ethers, alcohols and esters were prepared and tested as insecticides (STONECIPHER, 1951 and 1953). Chlorinated monocyclic terpenes and camphenes were also investigated during this period (STONECIPHER, 1951 and 1953) (BUNTIN, 1950, 1951, 1952, 1953 and 1965). Chlorinated camphene (later toxaphene), in 1944, was found to be insecticidal on houseflies (PARKER, 1947). Later, it was shown that it would be more effective on a wide range of cotton insects. Commercial production was started in 1947 and it was used worldwide, mostly on cotton; by 1979, 500 thousand tonnes of toxaphene had been sold (HOOPER, 1979).
This product is obtained by the chlorination of camphene to give a product with the empirical formula of C10H10Cl8 (Figure 2).
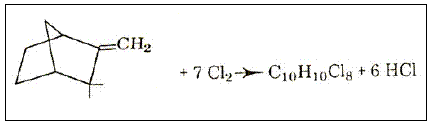
Figure 2 - Chlorination of camphene
Toxaphene is a quite readily soluble solid in organic solvents, especially in ketones and esters, and less so in alcohols and water.
Its insecticidal activity showed that it is effective in a range of 67-69% of toxaphene to 75% of housefly mortality.
Toxaphene is relatively stable at moderate temperatures but discolours and dehydrochlourinates at 110ºC.
Other chlorinated terpenes
Other commercial chlorinated terpene insecticides were made after the success of toxaphene. The first was Strobane, around 1955 (SCHULTZ, 1956). It was a chlorinated mixture of camphene and a-pinene containing 67% chlorine with about 2% a-pinene added as a stabilizer. It is a liquid with lower mammalian toxicity and it was also less toxic to insects.
In different parts of the world different chlorinated camphene was produced, using alternative methods of production.
The most important compounds of toxaphene are shown in Figures 3 and 4.
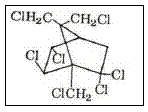
Figure 3 – The toxaphene B
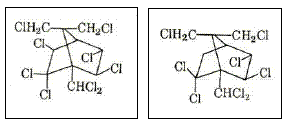
Figure 4 – Two other types of toxaphene
The toxicity of toxaphene as an insecticide is non-systemic; it has persistent contact and stomach toxicity. In animals, toxaphene may be absorbed through the skin, the lungs or the intestinal tract and the degree of absorption and toxicity depend upon the amount, the solvent and the route.
At the end of 1983, all labels and uses of toxaphene in the USA were cancelled, with a few exceptions. It may still be used in vat dips and spray dips on beef cattle and sheep for the control of scabies. In the Virgin Islands and Puerto Rico it may be used on pineapples and bananas for the control of mealy bugs and pine gummosis worms.
The chrysanthemumcarboxylic acids
By 1800, pyrethrum, a Persian insect powder, was known and used in the Caucasus as an insecticide (SHEPARD, 1951) and had probably been in use since antiquity. In 1858 it was in use in the USA. The powdered, dried flower beads of Chrysanthemum cinerariaefolium provide this insecticidal activity. This powder contains four insecticidal components which are esters of two cyclopropanocarboxylic acids with two cyclopentenone acids, pyrethrolone and cinerolone. The four esters have the following formula (Figure 5):
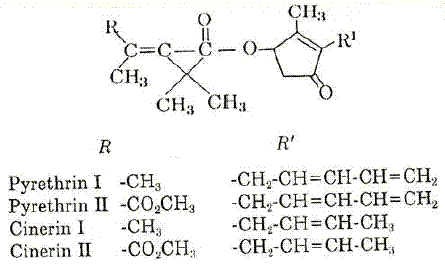
Figure 5 – Insecticidal components of pyrethrum
The two cyclopropane acids were identified as chrysanthemum-carboxylic acid (Figure 6a) and chrysanthemum-dicarboxylic acid (Figure 6b) (BURRESS, 1976).
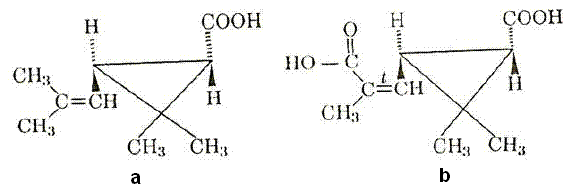
Figure 6 – Structure of chrysanthemum-carboxylic acid (a) and chrysanthemum-dicarboxylic acid (b)
The isomer of (+)-Trans-chrysanthemic acid is available from a-pinene or (+)-3-carene and also from limonene and a-terpineol (MATSUMURA, 1975).
While this acid can be synthesized from a-pinene or (+)-3-carene, it is made for commercial use by the combination of two isopropylidine moieties.
Terpenes and derivates
Over a period of millions of years there has been close association, evolution and adaptation between plants and insects. The plants have developed terpenes and their derivates for their own self-protection and interest (pollination). For the plants there are also repellents, attractants, anti-ingestants and in some cases physical traps to obtain food. The insects have reacted to these materials and ingested and use them or their biosynthesized derivatives for their own bio-regulation. They have used them as sex hormones, growth and development hormones and pheromones for behaviour such as feeding, aggregation, alarm, and defence.
The Ips species, major insect pests of conifers, are a good example of terpene use and adaptation. As an aggregation factor and sex attractant they evolved pheromones with some of the known components being 2-methyl-3-buten-2-ol (Figure 7), ipsdienol (Figure 8), (S)-cis-verbenol (Figure 9) and ipsenol (Figure 10) (BYERS, 1983 and 1990). A selective oxidation of a-pinene gives high yields of trans-verbenol, a compound useful as an aggregating pheromone for wood-boring insects (NOMURA, 1985).
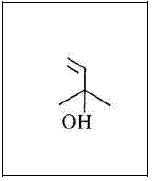
Figure 7 – The 2-methyl-3-buten-2-ol
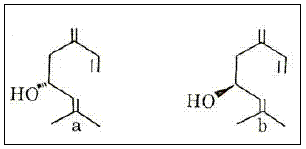
Figure 8 – The trans (a) and cis (b) ipsdienol
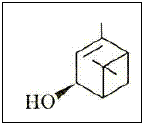
Figure 9 – The (S)-cis-verbenol
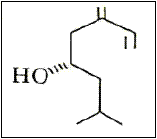
Figure 10 – The ipsenol
Racemic ipsdienol production has been seen in 83% yield from myrcene; that of R-(+)- and S-(+)-ipsdienol in yields of 70% and 60-65%, respectively.
The β-dialkyl-aminoalkyl ethers of terpene alcohols and their thio-ether analogues are also known to be herbicides as well as insecticides, repellents and synergists for pyrethrum (BORDENCA, 1968). The acyclic, monocyclic and bicyclic terpene alcohols are disclosed in this reference. N-Dialkyl-aminoalkoxy diterpenylamines and their thio-analogues are herbicidal, especially for aquatic weeds (BORDENCA, 1972);N-Dialkyl-aminoalkoxy alkyl terpenyla-mines are repellents for biting flies.
These are some of the known examples that show the presence of terpenes in repellents, insecticides, etc.
The turpentine residue (after pinene removal) containing 27-28% 3-carene, 20% dipentene, 2% β-myrcene, 8% a-pinene, 2% β-pinene and 3% camphene was an insecticide against forest insects (OSHKNEV, 1980). Mixtures of the same terpenes and in addition terpineol, terpinyl and bornyl acetates and terpinyl diacetate were insecticides and repellents (SMELYANETS, 1968) (KUZNETSOV, 1968). Bornyl esters (PERNG, 1979), menthyl acetate and other monocyclic and bicyclic terpene alcohol aliphatic and aromatic acid and di-acid esters are reported to be insecticidal (SVISHCHUK, 1974). Terpene alcohol N-methyl carbamates such as a-terpinyl N-methylcarbamates are also reported to be active as insecticides (GEARY, 1964). Terpene oxides (1,4- and 1,8-cineols) also have insecticidal activity (DASSLER, 1957); Geraniol (trans-3,7-dimethyl-2,6-oactdien-1-ol) and ethers of substituted (alkyl, halogen) phenols had juvenile hormone and insecticidal activity (MEL'NIKOV, 1979). Aromatic alcohols with terpene (geranyl) as a substituent alkyl group were acaricides (MYRSINA, 1978). Geranyl phenyl ethers (PALLOS, 1976), geranyl amines (SCHWARZ, 1975) and geranyl alkoxyalkyl ethers (BOWERS, 1977), all of which were epoxidised, were juvenile hormones and insecticides.
Limonene has also been registered as an insecticide (ANON., 1984; HEBEISH, 2008; MEYER, 1997).
Terpene polymers
Terpene Polymers are used as spreaders and stickers by addition to insecticide formulations (CLARK, 1967). These a- and β-pinene polymers also extend the life of volatile insecticides such as methyl parathion. Terpene phenol copolymers have been used with pyrethroids to provide controlled release of the active ingredient (JAPAN KOKAI TOKYO KOHO, 1983 and 1983a).
References
ANON., 1984. Chem. Week 134(26): 20. [ Links ]
BORDENCA, C., DERFER, J.M., KANE, B.J., 1968. South Africa Patent 67,07,649.
BORDENCA, C., 1972. U. S. Patent 3,703,554.
BORGLIN, J.N., 1940. U. S. Patent 2,209,184.
BORGLIN, J.N., 1940a. U. S. Patent 2,217,611.
BORGLIN, J.N., 1941. U. S. Patent 2,252,548.
BOWERS, W.S., 1977. U. S. Patent 4,014,901.
BUNTIN, G.A., 1950. U.S. Patent 2,514,564.
BUNTIN, G.A., 1951. U.S. Patent 2,579,296-301.
BUNTIN, G.A., LOHRG, A.D., 1952. U.S. Patent 2,594,526.
BUNTIN, G.A., 1953. U.S. Patent 2,667,164.
BUNTIN, G.A., 1965. U.S. Patent 3, 208,904.
BURRESS, R.M., GILDERHAUSS, P.A., CUMMING, K.B., 1976. Invest. Fish Control 1976, 77. [ Links ]
BYERS, J.A, 1983. Bark beetle conversion of a plant compound to a sex-specific inhibitor of Pheromone Attraction. Science, May 6, 220: 624-626. [ Links ]
BYERS, J.A., BIRGERSSON, G., 1990. Pheromone Production in a Bark Beetle Independent of Myrcene Precursor in Host Pine Species. Naturwissenschaften 77: 385-387. [ Links ]
CLARK, A.R., CLARK, M.M., 1967. U.S. Patent 3,314,981.
DASSLER, H.G., DUBE, G., 1957. Anz. Schadlingskunde 30, 86. [ Links ]
ENAN, E., 2001, Insecticidal activity of essential oils: octopaminergic sites of action. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, Issue 3, Nov., 130: 325-337 [ Links ]
HEBEISH, A., FOUDA, M.G., HAMDY, I.A., EL-SAWY, S.M., ABDEL-MOHDY, F.A., 2008. Preparation of Durable Insect Repellent Cotton Fabric: Limonene as Insecticide. Carbohydrate Polymers, Available online 29 February [ Links ]
HERCULES POWDER Co., 1954. Thanite an Insect Toxicant. Tech. Bull. 5M-7-5415443. [ Links ]
HOOPER, N.K., AMES, B.N. SALEH, M.A., CASIDA, J.E., 1979. Science 205, 591. [ Links ]
HUMPHREY, I.W., 1938. U.S. Patent 2,136,011
GEARY, R.J., 1964. U. S. Patent 3,123,525.
JAPAN KOKAI TOKKYO KOHO, 1983, Japan Patents 58.1113.102 (83,113,102).
JAPAN KOKAI TOKKYO KOHO, 1983a, Japan Patents 58.113.103 (83,113,103).
KUZNETSOV, N.V., RUDNEV, D.F., SMELYANETS, V.P., 1968, Dopov. Akad. Nauk. Ukr. RSR Ser. B, 30, 657. [ Links ]
MATSUMURA, F., HOWARD, R.W., NELSON, J.O., 1975. Chemosphere 4: 271. [ Links ]
MEL'NIKOV, N.N., YUDOVSKAYA, T.K., PRIDANTSEVA, E.A., ZHMAKINA, T.M., 1979. USSR Patent 529,760, C.A. 90: 35008u.
MEYER, TE, 1997. Corn rootworm control via formation of limonene in transgenic plants. Biofutur, Issue 164, Feb 1997, 1997: 43. [ Links ]
MYRSINA, R.A., PROTOPOPOVA, G.V., LAGOSHINA, N.S., 1978. Fiziol. Akt. Veshchestva 10: 63. [ Links ]
NOMURA, M., FUKIHARA, Y., 1985. Yukagaku 34: 467. [ Links ]
OSHKNEV, A. KH., STADNITDKU, G.V., 1980. Izv. Vyssh. Uchebn. Laved., Lesn. Zh. (6): 19. [ Links ]
PALLOS, F.M., TSENG, C.K., 1976. U.S. Patent 3,994,981.
PARKER, W.L., BEACHER J.R., 1947. Dela. Agr. Exp. Sta. Bulletin 264. [ Links ]
PIERPONT, R.L., 1939. Terpene effects in Pyrethrum and Rotenone Fly Sprays. Univ. Delaware Agr. Exp. Sta. Bulletin 217, Tech. Bulletin 24. [ Links ]
PIERPONT, R.L., 1941. J. Econ. Entom. 34, 195. [ Links ]
PERNG, T-H, LIU, L.-L., LEE K.-K., 1979, Tai-wan Yao Houch Tsa. Chih. 31(2): 119. [ Links ]
SCHULTZ, A.L., BLOOR, R.R., 1956. U.S. Patent 2,767,115.
SCHWARZ, M., SONNET, P.E., WAKABAYASHI, N., 1975. U. S. Patent 3,904,773.
SHEPARD, H.H., 1951, The Chemistry and Action of Insecticides, McGraw-Hill Book Co. Inc., New York. [ Links ]
SMELYANETS, V.P., KUZNETSOV, N.V., 1968. Khim. Sel. Khoz 6, 754. [ Links ]
STONECIPHER, W.D., 1951, U.S. Patent 2,546,174
STONECIPHER, W.D., 1953. U.S. Patent 2,657,166
SVISHCHUK, A.A., MAKHONVSKII, N.K., 1974. Fiziol. Akt. Veshchestva 6, 110. [ Links ]













