Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Silva Lusitana
versão impressa ISSN 0870-6352
Silva Lus. vol.19 n.Especial Lisboa 2011
Early Detection Methods for Pine Wood Nematode Infections of Maritime Pine in Portugal
Luis Bonifácio and Edmundo Sousa
INRB, IP – Instituto Nacional dos Recursos Biológicos. Quinta do Marquês, Av. da República, 2780-159 OEIRAS
Abstract
Four different techniques for early detection of pines infected by the pine wood nematode (Bursaphelenchus xylophilus) were tested in the maritime pine within the affected zone of Setúbal peninsula, in Portugal. From the four methods tested, the wood electrical resistance only had significantly different readings from healthy pines when the tree was already visually decayed. The sap flow measurements made in six previously selected pines didn't allow any conclusion, because none of the pines died during the survey. The oleoresin flow, induced using a punch hole in the trunk, with 14cm diameter, at 1.30m height, proved to be reliable for detecting an infected pine, when there is a complete absence of resin production (99% of the observations). The flow ceases about one and a half months before decay symptoms are obvious (when over 25% of the pine's needles are yellow).
Key-words: Pine wilt disease; maritime pine; oleoresin flow.
Métodos para a Deteção Precoce das Infeções pelo Nemátodo da Madeira do Pinheiro nos Povoamentos de Pinheiro Bravo, em Portugal
Sumário
Foram avaliados quatro técnicas como métodos para a deteção precoce da infecção de pinheiro bravo pelo nemátodo da madeira do pinheiro (Bursaphelenchus xylophilus), na zona afetada da península de Setúbal, em Portugal. A medição da resistência elétrica da madeira dos pinheiros apenas apresentou resultados significativamente diferentes entre os pinheiros saudáveis e os infetados quando estes já se encontravam com sintomas óbvios de declínio (copa consideravelmente amarelada). Os resultados obtidos das medições do fluxo de seiva, efetuadas em seis pinheiros previamente selecionados, foram inconclusivos porque nenhum dos pinheiros foi naturalmente infetado. A indução do fluxo de resina no tronco dos pinheiros, efetuada com um vazadouro com 14 cm de diâmetro a 1,30 m de altura, foi eficaz na deteção precoce de pinheiros infetados, em 99% das situações em que a produção foi nula. A cessação na produção de resina ocorreu cerca de mês e meio antes do pinheiro evidenciar sintomas de declínio, com mais de 25% das agulhas amarelas.
Palavras-chave: Doença do nemátodo da madeira do pinheiro; pinheiro-bravo; resinagem
Introduction
The pine wood nematode (PWN) Bursaphelenchus xylophilus (Steiner & Bührer) Nickle is the causal agent of the pine wilt disease that was detected in Portugal in 1999 (the first time in Europe), in a dead maritime pine Pinus pinaster Aiton, at Pegões, Setúbal peninsula (MOTA et al., 1999).
To infect a healthy pine the nematode needs to be transported by an insect vector, usually belonging to the genus Monochamus (LINIT, 1987; KISHI, 1995); in Portugal the only known vector is the native, secondary forest insect, Monochamus galloprovincialis (Olivier) (SOUSA et al., 2001).
Since it was detected, enormous efforts have been made to control the disease, namely: cutting and destroying infested pine trees before the exit of the insect vector and the use of traps lured with semiochemicals to capture de insect vector during its flight period. For all the measures the development of an in situ and real time method for early detection of the pines killed by the nematode would be an important stand management tool and to assess the real impact of the nematode in the overall mortality of the pines, allowing the separation of those trees from scolitids and other mortality causes.
For this purpose three different techniques based on the monitoring of the pines' physiological parameters were tested on about 400 maritime pine trees in two plots delimited inside the PWN affected area of Setúbal peninsula: oleoresin flow, sap flow, the wood's electrical resistance and comparison with visual canopy symptoms.
Materials and methods
To better understand evolution of the maritime pine decline associated with the Pine Wood Nematode infection, two plots with about 200 pine trees were established inside the initially affected area, at Tróia (Grândola, Setúbal) and Companhia das Lezírias (Samora Correia, Benavente). The objective was to test early infection detection methods, from the beginning of 2001 until the end of 2004.
Each tree in the plots was evaluated monthly, between April and September, for the oleoresin flow and visual decline symptoms. Afterwards, between October and April of the following year, visual evaluation was made bi-monthly. The visual evaluation of the sanitary condition of the pine trees was based on canopy discoloration, on a scale of 5 (from 0 – healthy to 5 – dead pine tree).
The first method tested was the measurement of the oleoresin flow. The flow was induced by a mechanical injury of the trunk at breast height (1.30m) using a 14cm diameter punch hole into the wood. To measure the oleoresin flow during 24 hours, a plastic tube was inserted, as described by TOGASHI (1988). The evaluation scale was based on MAMIYA (1983), where 1 – no flow; 2 – poor flow ( ≤ 5ml ); 3 – medium flow ( > 6ml e ≤ 10ml ); 4 – abundant flow ( > 11ml ) (Figure 1).

Figure 1 – Oleoresin flow mechanical induction on maritime pine inside experimental plots; (1) Incision on the trunk of 14 mm diameter; (2) Placement of the tube to collect resin flow; (3) Tube placed on healthy tree filled with resin
After the removal of the tubes the wound used was covered with a plastic substance, Arbokol (Quelmer), to prevent infections by biotic agents. The wound was only made in trees bigger than 15cm BHD, since it was considered that the continuous use of the procedure would kill smaller trees.
When a tree didn't show oleoresin production the wood was sampled at breast height (1.30 m) and taken to a laboratory at Oeiras for nematode extraction by the tray method (an adaptation of the Bearmann funnel) (PENAS et al., 2002) and for identification.
The second method was tested between April and October 2004. The electrical conductivity of the trunk of all trees in the plots was measured at the four main directions in each tree, every 2 weeks, to detect xylem cavitations. Trees with very small diameters (<15cm) only allowed one measurement, taken on the north side of the trunk. The device was connected by a flexible cable to a cylindrical handle with two metallic 10 cm needle probes. The probes were plastic isolated and the electrical resistance was measured by the last uncovered centimetre of the probes, when pushed deep inside the wood of the standing pine trees, at breast height (Figure 2). This kind of isolated probes is best for readings in woods with marked differences between the surface and the inside, likely to have higher moisture content (JAMES, 1988).
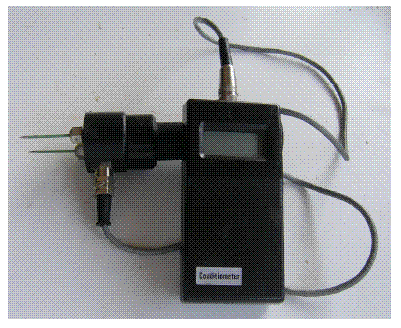
Figure 2 – Devise used to measure wood electric resistance of the pines inside the plots
The third method was to check if infection by the Pine Wood Nematode leads to the sudden interruption of sap flow. Six pine trees were chosen in a Tróia plot for the sap flow survey. The trees were representative of the stand (GRANIER et al., 2000) and attending to their size and placement in the plot, they were likely to be selected by the nematode vector and therefore of high risk of infection.
Sap flow was monitored during spring and summer of 2004 and 2005, which was the fourth early detection method. The pine trees were partially debarked and in each, two 2mm holes were drilled to place the sensors into the xylem, with the heated probe placed above and the other kept at inner wood temperature (reference probe). After the placement of the probes, that part of the trunk was carefully isolated to avoid animals, rain and direct solar radiation (Figure 3).

Figure 3 – Sap flow measurement in maritime pine inside Pine Wood Nematode survey plot during 2004 and 2005; (a) placement of probes; (b) pines with sap flow probes; (c) solar panel
The sap flow K was calculated based on the temperature difference detected by the probes and related empirically to the average sap flow speed (PERÄMÄKI et al., 2001). The probes were connected to a Data-Logger (Campbell Scientific) programmed for readings every 10 minutes. A solar panel was placed nearby to provide electricity to heat the probes.
All parametric and non-parametric statistical analysis was done using the Statistica package (version 6.1 – Statsoft Inc., 2003).
Results
Among all the methods tested, the oleoresin flow was the most accurate for early detection of declining pine trees. All pine trees that didn't show any change in the oleoresin flow continued healthy during the whole study period. From those which showed a reduction in production, only four percent died, while they died in 99% of the occasions (115 out of 123 method applications) after failing completely to produce any resin (Figure 4).

Figure 4 – Results of the mechanically induced oleoresin flow of the pine trees with Breast High Diameter over 15cm, at the plots in Tróia (a) and Companhia das Lezírias (b)
The assessment of crown discoloration of the pines confirmed the gradual swift from green to yellow and brown, as a visual effect of the attack by biotic agents, such as the PWN or scolitids. Nevertheless, on several occasions the slight yellowing (up to 10% of the crown) might not indicate a decline situation, and the pine remained healthy (Figure 5).
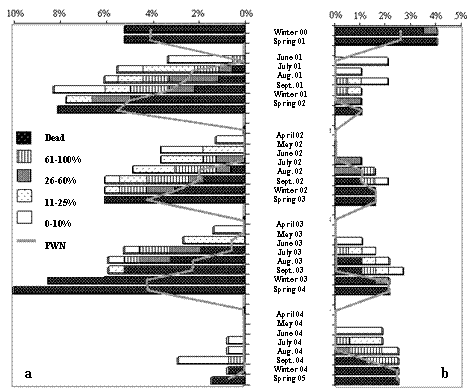
Figure 5 – Pine trees canopy visual discoloration and presence of Pine Wood Nematode in the trunk wood samples, collected at 1.30 m height, at Tróia (a) and Companhia das Lezírias (b)
The period between the time when the pines’ resin flow stopped and the occurrence of visual changes in the canopy was quite uniform during this study, without significant differences either between the plots, at Tróia and Companhia das Lezírias (F(1.39)= 0.83; p= 0.3666), nor between tree breast height diameter DAP (F(3.37)= 0.29; p= 0.8322), nor between the years of this study (F(3.37)= 0.69, p= 0.5615).
Therefore, it can be stated that the oleoresin flow stopped one month and a half, on average, before the yellowing was noticed in at least 25% of the crown, which occurred usually after June (Figure 6).
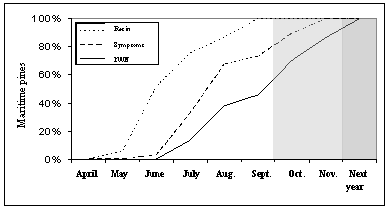
Figure 6 – Occurrence of oleoresin flow failure, visual discoloration of canopy and Pine Wood Nematode (Bursaphelenchus xylophilus) detection in wood samples, of the pines in the plots of Tróia and Companhia das Lezírias. Shade represent the months where the insect vector Monochamus galloprovincialis stops flying
Overall, the PWN was never detected in the wood sample collected at 1.30m, when the pine first stopped producing oleoresin. The later in the year oleoresin flow stopped, the later the infections and then the time period necessary to have a wood sample with PWN at breast height was bigger. At the end, the nematode was found in 68% of the pines that died during this study (34 PWN infected out of the total of 50 dead trees).
The appearance of visual symptoms occurred much earlier than PWN was found in the wood samples (Table 1).
Table 1 – Time period between the appearance of visual symptoms (yellow needles) and the detection of Pine Wood Nematode (Bursaphelenchus xylophilus) in the samples collected at 1.30 m high of the trunk of pine trees after oleoresin flow has stopped (average ± standard deviation), in days

In the previous table only PWN infected pines were considered, however, it is important to remember that visual symptoms considered in this study are not exclusive for PWN infections and are identical for pines attacked by other biotic agents (i.e. scolitids). In fact, no significant difference was found in the period of time between the oleoresin flow stop and visual symptoms detection for PWN infected trees and trees killed by other agents: F(1.39)= 0.41; p= 0.5246.
More than 9.000 measurements of wood electrical resistance were obtained from both plots.
ANOVA statistical analysis revealed significant differences between measurements according to directions (F(3.9245)= 36.78; p< 0.0001) with higher values at North. Nevertheless, the following analyses were based on an average of the four measurements taken from each tree. Statistical differences were found between plots (F(1.2480)= 293.18; p< 0.0001) with higher values at Companhia das Lezirias; this was dependent on pine dimensions (F(1.2477)= 170.57, p< 0.0001) with smaller readings in the bigger pines and between months. For this last comparison, Post-hoc Tukey test group showed early April and May to have the lowest readings, followed by late August and September, with higher electrical resistance in mid June and July (F(1.2473)= 36.33, p< 0.0001).
Most important was the significant difference found between healthy pines and those that showed yellowish needles.
The sap flow measured between May and September of 2004 and 2005 at the Tróia plot revealed a decrease along both years as weather got warmer and soil moisture decreased (Figure 7). Daily variation of the readings revealed higher sap flow during the day and a decrease towards sunset.
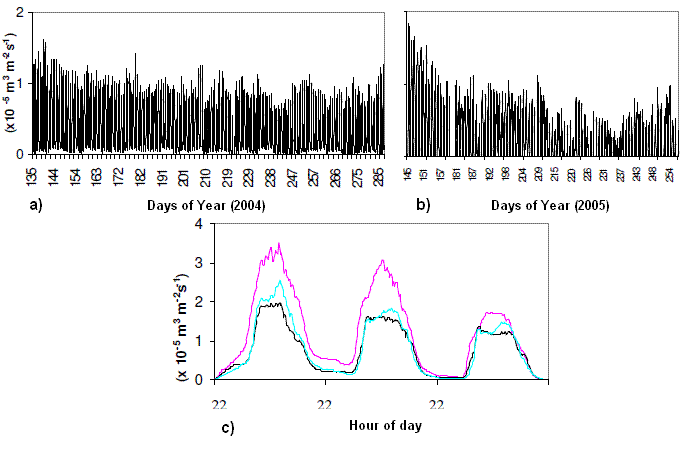
Figure 7 – Sap flow variation on the pine trees surveyed during 2004 and 2005, at Tróia plot. a) overall variation in one pine during 2004; b) overall variation in one pine during 2005; c) Daily variation in three maritime pines
None of the selected pines (as with no other pines in the plots) was infected by PWN during our two years, so it was impossible to fulfill the purpose of our study.
Discussion
Of all the methods tested for early detection of maritime pine infections by the pine wood nematode B. xylophilus, the oleoresin flow was found to be very reliable, as described for other pests (i.e. LORIO, 1994; ROCCHINI et al.,2000). This method’s overall accuracy was over 90% at Companhia das Lezírias and reached 96% at the Tróia plot, values identical to the work of MAMIYA (1983) in P. densiflora, in Japan.
Oleoresin production by the trees is, on most occasions, triggered by aggressions such as fire, mechanical wounds or fungi inoculation (RAFFA, 1991; LOMBARDERO et al., 2006) and also negatively affects the reproductive success of many species of scolitids (PHILIPS and CROTEAU, 1999; TAKASU et al., 2000; FADY et al., 2004). However, the oleoresin flow is not able to prevent infection by the nematode and moreover the first effect of the nematode is to stop the oleoresin production.
The oleoresin production obtained in healthy pines (over 11ml/day/tree) was greater than that described by LINIT et al. (1993) for P. taeda (3.5ml/day/tree), although the injury made by the authors was smaller (2.5cm diameter). This reveals the great oleoresin production potential of maritime pine for comercial proposes (THEMUDO and CARNEIRO, 1958; MENDES, 2004; PALMA, 2007).
For monitoring, the punch hole should be replaced by a pin, applied after debarking a small area. If the pine is healthy then an oleoresin drop should appear within an hour.
The visual symptoms of decline (yellowing of needles) related to pine wood nematode infection in maritime pine occurred, on average, after one and a half months after failure of oleoresin, apparently taking longer to develop than the three weeks observed in other pine species such as P. clausa var. clausa, P. elliotti var. elliotti (BLAKESLEE et al., 1987), P. sylvestris (MALEK and APPLEBY, 1984) and P. thunbergii (TOGASHI, 1989).
The time gap after oleoresin failure is bigger when the visual symptoms appear after the beginning of autumn, when M. galloprovincialis stops flying (NAVES et al., 2008); this is due to late infections. This fact is related to the decrease of temperature that becomes unfavourable to nematode reproduction and movement within the host tree (MAMIYA, 1984).
Overall, sap flow was very small because trees had small crowns and those years (mainly 2005) were very hot and dry. The maximum sap flow velocity obtained in healthy Tróia pines (3,5x10-5m3m-2s-1) was similar to those reported for P. sylvestris (3.0 x 10-5m3m-2s-1) (GRANIER et al., 1996; KÖSTNER, et al., 1998), less than those presented by LUNDBLADT et al. (2001) for Sweden, but higher than in Spanish Pyrenees mountains (6.13 x 10-6m3m-2s-1) (POYATOS et al., 2005) or Czech Republic (2.2 x 10-9m3m-2s-1) (CERMAK, et al., 2004).
Different values can be due to different tree species (no values are available for maritime pine), climatic conditions and time of year of the studies. If we look at sap flow on hot days at Tróia, much smaller values were obtained (about 1.0 x 10-5m3m-2s-1). If some of the surveyed pines were to be infected by PWN, then a strong reduction of the sap flow would be expected before visual symptoms, as occurred for scolitid attacks (BALLARD et al., 1982; BILLINGS and WARD, 1984; WULLSCHLEGER et al., 2004).
However this method cannot be used for early detection of PWN infections, because differences in readings were only found after visual symptoms were already obvious.
The wood electrical resistance cannot be used as an early detection method because significant differences in the readings were only obtained when declining pines already showed a considerable amount of yellowish needles. Other authors describe correlations between changes in wood electrical conductivity and susceptibility to pests (BALL and SIMMONS, 1984) or fungal diseases (TIITTA et al., 2003).
Differences observed in the readings between smaller and larger trees are due to the size of the wood vessels (MENCUCCINI et al., 1997; CARLL and TenWolde, 1996).
For climatic conditions of the affected area in Setúbal peninsula, pine wilt disease reveals itself as yellowish needles one and a half months after the pine stops oleoresin flow and another month is required for the tree’s crown to turn completely brown and the tree dies.
References
BALL, J.J., SIMMONS, G.A., 1984. The shigometer as predictor of bronze birch borer risk. J. Arboriculture 10(1/2): 328-331. [ Links ]
BALLARD, R.G., WALSH, M.A., COLE, W.E., 1982. Blue-stain fungi in xylem of lodgepole pine - A light microscope study on extent of hyphal distribution. Can. J. Bot. 60: 2334–2341. [ Links ]
BILLINGS, R.F., WARD, J.D., 1984 How to conduct a southern pine beetle aerial detection survey. Tex. For. Serv. Circ. 267: 12 pp. [ Links ]
BLAKESLEE, G.M., MILLER, T., ESSER, R.P., 1987. Observations on pine wood nematode-related mortality of sand and alsh pine seed orchard trees in Florida. In Wingfield ed. Pathogenicity of the Pine Wood Nematode pp. 40-45. [ Links ]
CARLL, C., TenWolde, A., 1996. Accuracy of Wood Resistance Sensors for Measurement of Humidity. J. Testing Evaluation 24(3): 154-160. [ Links ]
ČERMÁK, J., KUČERA, J., NADEZHD, N., 2004. Sap flow measurements with some thermodynamic methods, flow integration within trees and scaling up from sample trees to entire forest stands. Trees 18: 529-546. [ Links ]
FADY, B., FINESCHI, S., VENDRAMIN, 2004. EUFORGEN Technical Guidelines for genetic conservation and use for Italian stone pine (Pinus pinea). International Plant Genetic Resources Institute, Rome, Italy, 6 pp. [ Links ]
GRANIER, A., BIRON,P., KÖSTNER, B., GAY, L.W., NAJJAR, G., 1996. Comparisons of xylem sap flow and water vapour flux at the stand level and derivation of canopy conductance for Scots pine. Theor. Appl. Climat. 53: 115-122. [ Links ]
GRANIER, A., LOUSTAU, D., BRÉDA, N., 2000. A generic model of forest canopy conductance dependent on climate, soil water availability and leaf area index. Ann. For. Sci. 57: 755-765. [ Links ]
JAMES, W.L. (1988) – Electric moisture meters for wood. U.S.D.A. General Techn. Rep. 6: 17pp. [ Links ]
KISHI, Y., 1995. Pine wood nematode and the Japanese pine sawyer. Thomas Comp. Limited. Tokyo, Japan, 302 pp. [ Links ]
KÖSTNER, B., GRANIER, A., CŸERMAK, J., 1998. Sapflow measurements in forest stands: methods and uncertainties. Ann. Sci. For. 55: 13-27.
LINIT, M.J., 1987. The insect component of the pine wilt disease in the United States. in Wingfield ed. Pathogenicity of the pine wood nematode pp. 26-39. [ Links ]
LINIT, M.J., KONDO, E., SMITH, M.T., 1983. Insects associated with the pinewood nematode Bursaphelenchus xylophilus (Nematoda: Aphelenchoidae), in Missouri. Environ. Entomol. 12(2): 467-470. [ Links ]
LOMBARDERO, M.J., AYRES, M.P., AYRES, B.D., 2006. Effects of fire and mechanical wounding on Pinus resinosa resin defenses, beetle attack, and pathogens. For. Ecol. Manag. 225: 349-358. [ Links ]
LORIO, P.L., 1994. The Relationship of Oleoresin Exudation Pressure (or Lack Thereof) to Flow from Wounds. J. Sustainable For. 1(4): 81-93. [ Links ]
LUNDBLAD, M., LAGERGREN, F., LINDROTH, A., 2001. Evaluation of heat balance and heat dissipation methods for sapflow measurements in pine and spruce. Ann. For. Sci. 58: 625–638. [ Links ]
MALEK, R.B., APPLEBY, J.E., 1984. Epidemiology of pine wilt in Illinois. Plant Disease 68(3): 180-186. [ Links ]
MAMIYA, Y., 1983. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Ann. Rev. Phytopathol 21: 201-220. [ Links ]
MAMIYA, Y., 1984. The pine wood nematode. In Nickle WR (ed). Plant and insect nematodes. pp. 589-626, New York, Marcel Dekker. [ Links ]
MENCUCCINI, M., GRACE, J., FIORAVANTI, M., 1997. Biomechanical and hydraulic determinants of tree structure in Scots pine: anatomical characteristics. Tree Phys. 17: 105-113. [ Links ]
MENDES, A.M.S.C., FELICIANO, D., TAVARES, M., DIAS, R., 2004. The Portuguese forests. Country report delivered to the EFFE Project "Evaluating financing of forestry in Europe". Port. Catholic Univ. Porto, 226 pp. [ Links ]
MOTA, M.M., BRAASCH, H., BRAVO, M.A., PENAS, A.C., BURGERMEISTER, W., METGE, K., SOUSA, E., 1999. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1(7-8): 727-734. [ Links ]
NAVES, P.M., SOUSA, E., RODRIGUES, J.M., 2008. Biology of Monochamus galloprovincialis (Coleoptera, Ceerambycidae) in the Pine Wilt Disease affected zone, southern Portugal. Silva Lusitana 16(2): 133-148. [ Links ]
PALMA, M.A.V., 2007. Capacidade produtiva de resina do pinheiro bravo. Brave panorâmica do sector resineiro em Portugal. Monografia apresentada para acesso à categoria de Investigador Auxiliar. I.N.R.B./E.F.N. Oeiras, 96 pp. [ Links ]
PENAS, A.C., DIAS, L.S., MOTA, M.M., 2002. Precision and selection of extraction methods of Aphelenchid nematodes from maritime pine wood, Pinus pinaster. J. Nematology 34(1): 62-65. [ Links ]
PERÄMÄKI, M., NIKINMAA, E., SEVANTO, S., ILVESNIEMI, H., SIIVOLA, E., HARI, P., VESALA, T., 2001. Tree stem diameter variations and transpiration in Scots pine: an analysis using a dynamic sap flow model. Tree Physiology 21: 889-897. [ Links ]
PHILLIPS, M.A., CROTEAU, R.B., 1999. Resin-based defences in conifers. Trend in Plant Sci. 4(5): 184-190. [ Links ]
POYATOS, R., LLORENS, P., GALLART, F., 2005. Transpiration of montane Pinus sylvestris L. and Quercus pubescens Willd. forest stands measured with sap flow sensors in NE Spain. Hydrol. Earth Syst. Sci. 9: 493–505. [ Links ]
RAFFA, K.F., 1991. Induced defensive reactions in conifer-bark beetle systems. in Tallamy & Raupp eds. Phytochemical Induction by Herbivores pp. 245-276. [ Links ]
ROCCHINI, L.A., LINDGREN, B.S., BENNETT, R.G., 2000. Effects of resin flow and monoterpen composition on susceptibility of lodgepole pine to attack by the Douglas-fir pitch moth, Synanthedon novaroensis (Lep.; Sesiidae). J. Appl. Ent. 124: 87-92. [ Links ]
SOUSA, E., BRAVO, M.A., PIRES, J., NAVES, P., PENAS, A.C., BONIFÁCIO, L., MOTA, M., 2001. – Bursaphelenchus xylophilus (Nematoda; Aphelenchoididae) associated with Monochamus galloprovincialis (Coleoptera; Cerambycidae) in Portugal. Nematology 3(1): 89-91. [ Links ]
TAKASU, F., YAMAMOTO, N., KAWASAKI, K., TOGASHI, K., KISHI, Y., SHIGESADA, N., 2000. Modelling the expansion of an introduced tree disease. Biol. Invasions 2: 141-150. [ Links ]
THEMUDO, J.C.F., CARNEIRO, A., 1958. A resina, suas vantagens e inconvenientes. Aspectos técnicos e económicos. Min. Economia/Junta Nac. Resinosos, Lisboa. [ Links ]
TIITTA, M., KAIKULAINEN, P., HARJU, A.M., VENÄLÄINEN, M., MANNINEN, A.M., VUORINEN, M., VIITANEN, 2003. Comparing the effect of chemical and physical properties on complex electrical impedance of scots pine wood. Holzforschung 57(4): 433-439. [ Links ]
TOGASHI, K., 1989. Variation in external symptom development of pine wilt disease in field grown Pinus thunbergii. J.Jpn. For. Soc. 71(11): 442-448. [ Links ]
WULLSCHLEGER, S.D., MCLAUGHLIN, S.B., AYRES, M.P., 2004. High-resolution analysis of stem increment and sap flow for loblolly pine trees attacked by southern pine beetle. Can. J. For. Res. 34: 2387-2393. [ Links ]
Acknowledgements
The work presented was partially funded by the European Union Fifth Framework project QLK5-CT-2002-00672—PHRAME (Development of improved pest risk analysis techniques for quarantine pests, using pinewood nematode, B. xylophilus, in Portugal as a model system). Our thanks to: Dr. Christian Tomiczek for making the wood conductivity resistance measurement device available, Eng. Célia Ferreira of SONAE Tourism Enterprises and Eng. Rui Alves of Companhia das Lezírias, for all their assistance in establishing and surveying the plots, at Tróia and Companhia das Lezírias.













