Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Corrosão e Protecção de Materiais
versão On-line ISSN 2182-6587
Corros. Prot. Mater. vol.33 no.3 Lisboa set. 2014
ARTIGO
Corrosion of hot-dip galvanized steel reinforcement
Corrosão do aço galvanizado
R. B. Figueira*1,2, C. J. R. Silva2, E. V. Pereira1 and M. M. Salta1
(1)LNEC, Laboratório Nacional de Engenharia Civil, Av. do Brasil 101, 1700-066 Lisboa, Portugal, e-mails: rmfigueira@lnec.pt, epereira@lnec.pt, msalta@lnec.pt
(2) Centro de Química, Universidade do Minho, Campus de Gualtar, 4710-057 Braga, Portugal, e-mail: csilva@quimica.uminho.pt
(*) A quem a correspondência deve ser dirigida, e-mail: rmfigueira@lnec.pt
ABSTRACT
In the past several years hot-dip galvanized steel (HDGS) has successfully been used to extend the service life of reinforced concrete structures, mainly due to its low cost and high corrosion resistance compared to mild steel. However, the initial corrosion behaviour of the galvanized coating when embedded in concrete and how that behaviour may be affected by the surface composition of the coating remains unclear. When HDGS is embedded in fresh concrete (a highly alkaline environment), the zinc coating corrodes vigorously until passivation occurs and the concrete hardens. This leads to two main concerns, namely, the high initial corrosion rate, which may remove so much zinc that this leads to insufficient galvanic protection of the underlying steel, and the hydrogen production (in the corresponding cathodic half-cell reaction) which may increase the porosity of the adjacent cement paste and reduce the bond strength between the bar and the concrete. To avoid, or at least minimize, this initial reaction the use of chromates has been implemented. However, due to the toxicity of hexavalent chromium, environmental and human health concerns have restrained their use and alternatives are being studied all over the world. In this paper, the coatings and pre-treatments studied at an academic level in the last few years (2001-2014) have been reviewed together with the corrosion mechanisms of HDGS in contact with high alkaline environments and the role of Cr(VI) in inhibiting the initial zinc corrosion process.
Keywords: Hot-Dip Galvanized Steel, Corrosion, Coatings, Pretreatments
RESUMO
A crescente utilização aço galvanizado em estruturas de betão armado deve-se essencialmente ao seu baixo custo e à melhoria da resistência à corrosão quando comparado com o aço sem proteção. A composição e morfologia do revestimento de zinco é determinante para o comportamento observado nos primeiros instantes de contacto do aço galvanizado com o betão fresco, dada a natureza fortemente alcalina deste meio. Durante o processo de cura do betão, o revestimento de zinco corrói-se vigorosamente até que a passivação ocorra. Nesta fase inicial, a elevada velocidade de corrosão do zinco pode contribuir para remover uma quantidade significativa deste revestimento originando uma proteção insuficiente do aço subjacente. Em simultâneo ocorre a produção de hidrogénio (resultante da reação catódica) a qual pode originar um aumento da porosidade do betão que por sua vez pode comprometer a aderência entre as armaduras galvanizadas e o betão.
Para se minimizar esta reação inicial, entre o aço galvanizado e o betão fresco, a aplicação de cromatos foi um procedimento amplamente utilizado quer como pré-tratamento do substrato metálico em aço galvanizado quer como componente do cimento utilizado. Contudo, a elevada toxicidade do crómio hexavalente e as nefastas consequências para a saúde humana e impacto no ambiente, resultaram na implementação de legislação restringindo severamente a sua utilização. Dada a sua elevada importância como pré-tratamento inibidor de corrosão, tem-se registado um crescente esforço na pesquisa de processos e materiais alternativos para tais fins. Neste artigo apresenta-se uma síntese dos resultados mais relevantes obtidos no estudo de revestimentos e pré-tratamentos destinados a aços galvanizados publicados no período que decorre entre 2001 e 2014. As soluções desenvolvidas para descrever os mecanismos de corrosão propostos na literatura, assim como a importância do Cr(VI) na inibição do processo inicial de corrosão do zinco em meios extremamente alcalinos são também apresentados e discutidos.
Palavras-chave:: Aço Galvanizado, Corrosão, Revestimentos, Prétratamentos
1. INTRODUCTION
The corrosion of steel in concrete is one of the major causes of structure degradation, requiring expensive rehabilitation. The estimated cost of corrosion damage of reinforced concrete bridges in the United States, due to the use of de-icing salts alone, was between $325 million and $1 billion per year [1]. In Australia, Europe and the Middle East the statistical results are similar [2]. Corrosion of the reinforcement in concrete is the main cause of the failures of these structures [2]. The most effective way to minimize the risk of reinforced concrete (RC) corrosion is to ensure that the cover of the metallic reinforcement sections is of an adequate thickness and possesses a high concrete quality, with a proper mixing ratio, good compaction and curing. There are four essential materials in the production of concrete including: Portland cement, sand, aggregates (stone) and water. The ratio of aggregate and sand to cement is an essential factor in determining the compressive strength of concrete mixture. The ratio of these materials affects the production of concrete and is directly related to its performance. These two features combined cover thickness and good quality concrete; confer an excellent protection to the reinforcing steel from the environmental exposure. The concrete coating works as a physical barrier for protection against aggressive environmental agents that create conditions for the passivation of the steel.
Portlandite, the main constituent of the mineralogical components used for the fabrication of concrete, together with uncontaminated water and non-aggressive admixtures or aggregates, containing mostly K+, Na+, Ca2+, OH- ions and dissolved O2 (from atmospheric exposure), has an important role in the durability of the concrete as it is responsible for the high alkalinity of fresh concrete (pH = 13.5). When embedded in fresh concrete, steel is protected as the concrete provides a physical protection barrier and the high pH leads to the formation of a passivation film in the reinforced steel, protecting it from corrosion. However, during the process of hydration and hardening, tensile forces can be generated causing the formation of cracks (on concrete structures), leading to reduced efficiency of the physical barrier that protects the steel. The degradation of the concrete properties often results in a common action of external and internal factors. It is a complex process, largely determined by the physicochemical properties of the concrete (internal factor) and the atmosphere that it is exposed to (external factor). Moreover, the physical barrier provided by the concrete cover is not perfect due to the porous structure of concrete. The existence of imperfections during concreting and curing, and the conjugation of these two factors, enable the entrance (diffusion/transport) of aggressive species into the steel/concrete interface and may cause a rupture of the passivation film, therefore initiating the corrosion of the RC. The most frequent causes responsible for breaking down the passivation film are the inclusion of Cl- in the film as well as the reaction of atmospheric CO2 with the constituents of the concrete. The volume of corrosion products formed, due to the presence of aggressive species, is about 4 to 6 times higher than the steel. Therefore, the evolution of corrosion in RC structures causes forces of expansion in the vicinity of the metallic parts, which, if it exceeds the tensile strength of concrete, initially leads to cracking (Figure 1), and then spalling of the concrete cover.
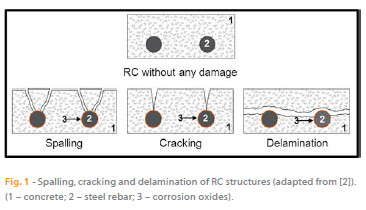
The continuing evolution of the corrosion of RC structures subsequently causes loss of adhesion between the concrete and steel construction, loss of steel ductility and section reduction of the steel rebar that may implicate the stability of the structure. The electrochemical process of corrosion on the steel surface, where both cathodic and anodic areas are located, is explained by the following reactions:
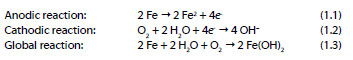
To increase the service life of RC structures, the use of hot-dip galvanized steel (HDGS) has been recognized as an effective measure [3, 4]. The main cause for the widespread use of galvanized steel is the dual nature coating. As a barrier coating, the layer formed by the galvanizing process offers a resistant and metallurgically bonded zinc coating that covers the steel surface, protecting it from the corrosive action. Moreover, the zincs sacrificial (cathodic) action protects the steel even when damage occurs in the concrete [3,4] and zinc corrosion products occupy a smaller volume than those produced from iron. Yeomans [3] confirmed that zinc corrosion products are powdery, non-adherent and capable of migrating from the surface of the galvanized reinforcement into the concrete matrix, reducing the likelihood of zinc corrosion-induced spalling of the concrete [3, 4]. Galvanized reinforced steel can withstand exposure to chloride ion concentrations several times higher (at least 4 to 5 times) than the chloride level that causes corrosion in steel reinforcement. Additionally, while steel in concrete typically depassivates below a pH of 11.5, galvanized reinforcement can remain passivated at a lower pH, thereby offering substantial protection against the effects of concrete carbonation [3]. The combination of these two factors, carbonation resistance and chloride tolerance, are commonly accepted as the basis for superior performance of galvanized reinforcement compared to steel reinforcement [5].
However, when HDGS is in contact with high alkaline environments, such as fresh concrete (pH = 13.5), the zinc corrodes and H2 evolution takes place. This initial corrosion process, extensively studied by several authors [6–8], may lead to zinc consumption until the formation of passivation layer (which passivates the steel reinforcement) or corrosion continues until all the zinc layer is consumed [6]. To avoid and minimize the H2 evolution, procedures such as increasing the chromate content of the cement, adding water-soluble chromates into the preparation and chromate conversion layers have been implemented and are capable of minimizing the initial zinc corrosion. However, due to the toxicity of Cr(VI) ions, environmental and human health concerns have restrained their use and alternatives are being studied all over the world.
In this paper, potential alternatives to the use of chromium-based compounds (Cr(VI)) published in the last few years (2001-2013), were reviewed. The corrosion mechanisms of HDGS in contact with high alkaline environments and the role of Cr(VI) in inhibiting the initial zinc corrosion process were also discussed.
2. HOT-DIP GALVANIZED STEEL (HDGS)
Hot dip galvanizing is the most important zinc coating process [4]. During this process, steel is immersed in a molten zinc bath at a temperature of 450 ºC. At this point, metallurgical interaction occurs between the iron and the molten zinc, forming an adherent coating that provides both an excellent barrier against iron corrosion. It also provides cathodic protection when imperfections exist or when there is local dissolution of the coating. The hot dip galvanizing process has been widely studied and detailed information can be found in the literature [4, 9 – 13]. Metallurgical reactions occur during the hot dip galvanizing process, leading to the formation of several intermetallic phases with increasing iron content. The intermetallic layers (from the inner phase of the substrate to the outer layer) include: ? (gamma phase), d (delta phase), ? (zeta phase) and ? (eta phase) as shown in Figure 2 [9].
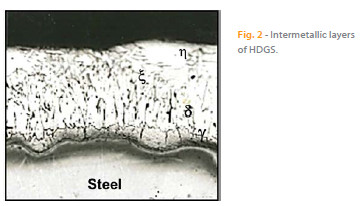
The gamma phase is a very thin layer with a cubic structure. The iron is present in a range between 21 % to 28 % of the layer and is often not easily identifiable. The delta layer is usually composed of two layers; an internal compact layer and an external barrier layer. The latter layer has a hexagonal structure and an iron percentage between 7 and 12 %. The zeta layer is formed by asymmetric monoclinic crystals, which is less rich in iron (the range may vary from 5.8 to 6.2 %). The last outer layer, eta is an external layer of almost pure zinc [9]. The composition and thickness of these intermetallic layers depends on several factors, such as the composition of the steel substrate and surface roughness, the molten zinc bath temperature, withdrawal speed from the molten zinc bath, composition of the zinc bath and the immersion time [4, 9].
The corrosion rate of zinc, in most natural environments, is 5 to 100 times slower than steel [14, 15]. To minimize these effects and increase the lifetime of steel, additional protection may be provided by the use of a zinc layer. The high corrosion resistance of galvanized steel is attributed both to the galvanic action of zinc and to the barrier effect of zinc products. Zinc has a low-self corrosion rate and, due to its low position in the galvanic series, is an efficient sacrificial anode for galvanic protection of steel structures [11, 14, 16].
Studies on the corrosion of galvanized steel have shown that this process involves three different stages [18 – 21] (Figure 3):
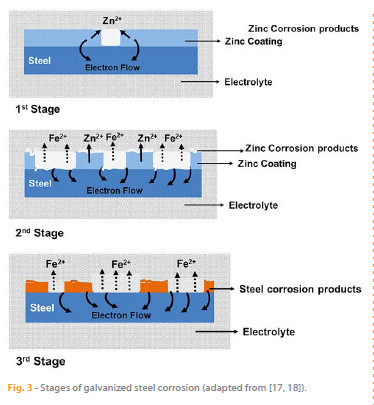
The first stage is mainly related to the dissolution of the oxide layer formed on the surface of the substrate during contact with air. The corrosion rate increases rapidly and the corrosion potential shifts towards less noble values (lower values) leading to the acceleration of the anodic process.
During the second stage, the surface of the zinc layer is covered with white rust (zinc oxide) due to partial/total dissolution of the zinc (depending on the electrolyte and the aggressive species present). Consequently, the corrosion rate decreases and the corrosion potential shifts to more noble values (higher values). In this stage, the dissolution of zinc is inhibited due to the presence of oxides and the underlying steel starts to corrode. In the third stage, the formation of red rust on the substrate surface increases. The corrosion rate remains constant and the corrosion potential continues to shift to higher values.
Even though some parts of zinc remain in the rebar, the galvanized steel shows a corrosion potential similar to that of carbon steel. At this stage, the underlying steel corrosion evolves due to the dissolution of iron and the zinc coating that remains (if no total dissolution occurred) no longer acts as sacrificial anode.
2.1 HDGS corrosion process in alkaline environments
Several studies have been carried out to understand the corrosion and passivation mechanisms of zinc in alkaline solutions. In 1964, Bird [22] showed that for a pH > 12.9 the main anodic product formed was - 22 ZnO . Lieber and Gebauer [23], in 1969, were the first to recognize the formation of calcium hydroxyzincate (CAHZ) as the corrosion product of zinc in alkaline solutions rich in calcium and that it was responsible for the passivation of the substrate. One year later, Rehm and Lämmke [24] published a paper that was consistent with the studies of Lieber and Gebauer. However, the authors proposed that before the formation of CAHZ, Zn(OH)2 was formed.
In 1972 Grauer and Kaesche [25] showed the formation of a ZnO layer in NaOH solutions and in the same year, Liebau et al. proposed the following mechanism [26]:
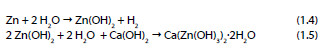
One year after, Shams El Din et al. [27] showed that when zinc is in contact with NaOH solutions with a concentration lower than 0.3 M, a progressive precipitation of Zn(OH)2 occurs. However, in solutions with concentrations higher than 0.3 M the zinc dioxide anion is formed as described in the following reaction:
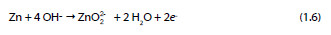
In 1974, Vorkapic et al. [28] studied the passivation of zinc in different concentrated solutions of KOH (1, 3, 6 and 10 M) and showed that the corrosion of zinc in the referred solutions is time dependent and a period of about 100 hours is necessary to reach a steady state. They also proposed and discussed a multi-step mechanism. Duval and Arliguie, in the same year, studied the possible reactions of zinc in contact with saturated solutions of calcium hydroxide. The influence of immersion time, surface conditions and temperature on the zinc samples was the focus of the study performed by the authors [29].
Zembura and Burzynska in 1977 [30] studied the corrosion of zinc in a pH range from 1.6 to 13.3 in de-aerated solutions 0.1 M of NaCl and found that for pH values above 11 the reaction is controlled diffusionally by the ions - 22 ZnO or.
In 1985, Baugh and Higginson [31] showed that zinc dissolves over an extensive range of electrochemical potentials and discussed the relationship of this behaviour to other metal/metal-ion systems. Two years later, Macías and Andrade [32, 33] studied the behaviour of galvanized steel in 0.001 - 1.5 M solutions of NaOH and KOH with or without calcium hydroxide and showed that in 12 < pH < 13.2 the zinc layer corrodes at a low rate. At pH < 12 only localized corrosion took place and for pH > 13.2 total dissolution of the zinc layer occurred without passivation. A threshold pH value was also found for the onset of H2 evolution, which was equal to 12.8. In the same year, Macías and Andrade also discussed the morphology and composition of the corrosion products on galvanized steel immersed in 0.05 - 1.5 M solutions of KOH.
Macias and Andrade also confirmed the results obtained by Lieber and Gebauer (formation of a passivation layer of CAHZ) and showed that the surface morphology of the HDGS was dependent on the pH of the solution where the corrosion process took place [7]. The same authors also confirmed the formation of ZnO and Zn(OH)2 corrosion products and it was shown that once the passive film of CAHZ was formed its stability remained even if the pH increased afterwards [7, 34].
As stated previously, several studies have been devoted to understanding the corrosion and passivation mechanisms of zinc in alkaline solutions [23, 26–28]. The most comprehensive work was performed by Andrade and co-workers [7, 8, 33–37].
Taking into consideration the results of various researchers, Andrade and Alonso [3] proposed a four step reaction mechanism to describe the corrosion process of zinc in strongly alkaline, calcium-containing solutions:
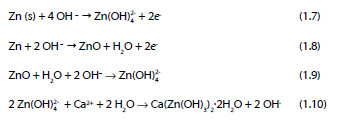
The morphology of the passivation layer of the corrosion products on galvanized steel substrates after being in contact with an alkaline solution are shown in Figure 4. It is reasonable to assume, that due to the size of the CAHZ crystals formed in solutions of 12.5 ± 0.1 < pH < 13.3 ± 0.1, the zinc surface is densely covered, providing full passivation of the substrate by the CAHZ crystals (Figure 4).

As the pH increases, the CAHZ crystal size increases, leading to crystals that do not completely cover the surface of the substrate (Figure 5). Under these circumstances, the passivation of the surface of the substrate is difficult to reach and zinc dissolution continues until all the zinc has dissolved.

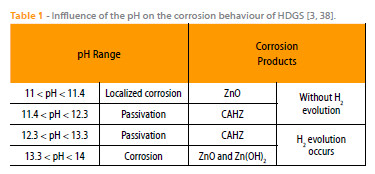
Table 1 is based on the information provided by Andrade and co-workers [3, 38] and briefly shows the influence of the pH on the corrosion products, whether hydrogen evolution occurs or not and if the substrate is locally attacked, corroded or passivated. Table 1 shows that for pH > 12.3 hydrogen evolution takes place and for 11.4 < pH < 13.3 the formation of CAHZ occurs.
2.2 HDGS corrosion process during concrete setting
Concrete is a highly alkaline environment due to the presence of calcium, sodium and potassium hydroxides (pH = 12.6) [3]. The pH increases to values above 13 in the first several hours of curing, reaching a maximum of 13.7 as hydration continues. Under these conditions, immediately after HDGS has been embedded in fresh concrete, and as the zinc is thermodynamically unstable at this pH, the zinc corrodes for a limited period (from several hours to a few days) until passivating surface layers are formed and the concrete hardens. Once the concrete sets, the hydrogen evolution ceases; the coating of CAHZ formed apparently provides protection to the reinforcement and no further attacks take place after this initial setting is over. Nevertheless, this initial corrosion process may lead to a consumption of the zinc layer of a thickness between 5 to 10 µm [9]. At the same time, hydrogen is produced which may cause the loss of adhesion between steel and concrete.
Several corrosion studies reported the behaviour of HDGS in contact with concrete media [3, 22, 39–48]. However, uncertainties concerning the initial corrosion behaviour of the galvanized coating when embedded in concrete still remains. The main literature about corrosion and passivation mechanisms of zinc in concrete environments, suggest that the formation of the protective layer due to zinc oxidation takes place with water reduction and subsequent hydrogen evolution [3, 6–8, 26, 32, 33, 38]. Other authors claim that the formation of protective layers is related to the presence of oxygen at the concrete/rebar interface [49, 50].
The chemical equations involved in the corrosion of zinc in concrete found in the literature are [45]:
Anode reaction:
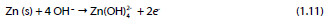
Cathode reaction:

Global reaction:
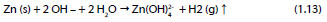
The conversion of zinc into CAZH is described by the reaction:
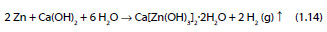
Additionally, the formation of zincate and hydrogen may occur as described by [45]:

Zincate ion further reacts with water leading to the formation of zinc hydroxide [45]:
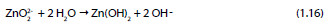
Studies regarding the effectiveness of the chromate treatments on HDGS when in contact with alkaline environments are limited. Short et al. [46, 51] showed that regardless of the chromate treatments on HDGS, the zinc coating is generally dissolved when exposed to alkaline solutions. This suggests that the CCCs may not be stable in highly alkaline solutions. On the other hand, researchers have been devoted to the study of the effectiveness of CCCs on corrosion resistance of HDGS in environments containing chlorides. In this case it has been unanimously shown that CCC improves the corrosion resistance of zinc [3, 42, 44, 45, 52–55]. Despite these contradictory findings, the galvanizing industry believes that CCCs are necessary to hinder the hydrogen evolution and minimize the initial zinc corrosion process in fresh concrete. Common procedures such as increasing the chromate content of the cement or adding water-soluble chromates into its preparation have been implemented to protect the galvanized rebars [3, 4]. The original reason behind the use of CCCs on galvanized steel is to avoid the formation of wet storage stain during the first six weeks after galvanizing, and in particular to reduce the formation of excessive amounts of zinc oxide and zinc hydroxide during that period [3].
The high corrosion resistance offered by the use of chromate compounds is due to the presence of Cr6+ and Cr3+. Chromate ions are oxidative inhibitors that hinder hydrogen evolution in fresh concrete, leading to the formation of a passive film of zinc chromate and chromic oxide [3, 45]. However, a controversial study was found [46]. The authors conclude that the chromate treatment was unnecessary and the chromate film appears to retard the formation of CAHZ and reduces the passivation of the zinc [46]. Other authors claim that the presence of chromium reduces the corrosion rate of HDGS and delays the passivation [3, 44]. Despite the differing conclusions on the use of chromates, it has been reported that chromate and similar hexavalent chromium compounds are toxic and carcinogenic. Their use in industry is a common source of serious environmental issues, which has led to their application being heavily regulated by most environmental legislation. Current commercial Portland cements have a limited content of Cr(VI) in their composition and the use of CCC is currently being avoided. Thus, intense research is being undertaken to replace chromates with more ecological compounds.
2.3 Environmentally friendly alternatives to the use of chromium based compounds
Many consider HDGS as the oldest and most economical method used for applying zinc coatings on steel [4, 56] . During the last few years, developments in this field have included new surface treatments, incorporation of composite materials into the bath and post-treatment techniques such as chromate and phosphating conversion layers. Table 2 shows the main surface treatments on zinc substrates or metallic substrates coated with zinc used in publications between 2001 and 2013 as well as the main conclusions/achievements. Studies that were performed in order to improve the process of the production of HDGS were not taken into consideration. However, several studies have been performed [56–66], such as Sere et al. in 1999 where results showed that the addition of small concentrations of lead and antimony improved the zinc coating uniformity and its adhesion to the steel substrate [67]. In 2007, Pistofidis et al. demonstrated that the incorporation of bismuth into the galvanizing bath, at certain proportions, lead to improved adhesion and corrosion resistance of the substrate [68]. The use of metal oxides, such as ZnO, ZrO2 and TiO2, as bath additives have also been reported [69-70].
The current alternatives to Cr(VI) show positive and negative performances when considering properties such as corrosion resistance, adhesion, fatigue resistance, reliability and quality control. Multiple studies have been undertaken to find alternatives in order to mitigate the harmful effects of an initial excessive reaction between the cement pastes and the zinc [71–73]. However, processes with similar performances as CCC with the same associated costs have not yet been achieved. Liu et al. in 2010 showed that molybdate-based CC provided a corrosion behaviour similar to the one offered by CCC however the authors also stated that the market cost of the molybdate based solution was much higher than the chromate solution [74]. An innovative study on the incorporation of silanes in the concrete admixture to protect the HDGS in RC structures was also reported [75] and the authors concluded that hydrophobic concrete protected the substrate in RC structures in the presence of cracks.
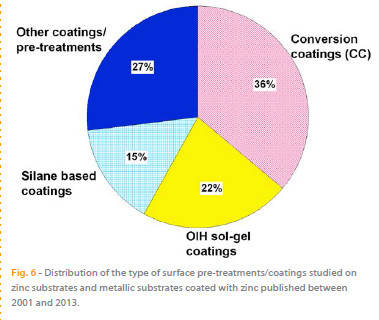
The analysis of Table 2 and Figure 6 shows that the main efforts for finding new and efficient pre-treatments to minimize the corrosion of zinc substrates were focused on testing and producing new CCs applied directly on the HDGS surface, representing 36 % of the published papers mentioned. The analysis shows that the number of published papers increased significantly after 2006. This growth is closely related with the Directive 2002/95/EC on the Restriction of Hazardous Substances that was adopted in February 2003 by the European Union and took effect in July 2006.
3. FUTURE AND RESEARCH CHALENGES ON CORROSION OF HOTDIP GALVANIZED STEEL REINFORCEMENT
According to Andrade [35], studies regarding the corrosion of RC structures started in the early 70s. During the last five decades, numerous papers have been published on this subject. However, due to controversial studies and contradictory results [3, 44, 46, 135] further studies on the role of hexavalent chromium in hindering the hydrogen evolution should be performed.
The analysis of the papers published and referred to in this article have also shown that molybdate based conversion coatings are very promising, displaying behaviour very similar to CCC. The negative aspect of this pre-treatment is that the associated cost is higher than for CCC. Therefore, the production of effective molybdate CC with lower concentration of Molybdate (to minimize the costs), is a path that will most certainly be explored.
OIH coatings prepared by sol-gel process have also been used as potential corrosion protection of HDGS. The unique properties of OIHs allow new end-use applications and display a significantly better cost/performance relationship when compared with other available materials in the marketplace. Research on contemporaneous hybrid polymers has been gaining market niches, which leads the authors to believe that these materials are also another path for future success. All innovative materials, and OIHs are no exception, face many difficulties and challenges for large-scale industrial production. Several factors including capital investement, ease of manufacturing, coating performances and environment issues need to be considered when developing a coating process for industrial application.
It is undeniable that future trends for coating systems need to be low cost, pollution-free, and easy to synthesize and actually be effective in preventing, minimizing and controlling the corrosion of the metallic substrates. Combined systems including the so-called green smart materials will be developed allowing for the capacity of regeneration and react according to the needs of the medium surrounding the substrate.
Acknowledgements
The authors would like to gratefully acknowledge the financial support from Fundação para a Ciência e Tecnologia (FCT) for the PhD grant SFRH/BD/62601/2009 and the financial support by Centro de Química [project F-COMP-01-0124-FEDER-022716 (ref. FCT Pest-/Qui/ UI0686/2011)-FEDER-COMPETE].
REFERENCES
[1] National Research Council (U.S.), Committee on the Comparative Costs of Rock Salt and Calcium Magnesium Acetate (CMA) for Highway Deicing, Highway deicing: comparing salt and calcium magnesium acetate, Transportation Research Board, National Research Council, Washington, D.C., 1991. [ Links ]
[2] H. Böhni, Corrosion in Reinforced Concrete Structures, H. Böhni, Woodhead Publishing Ltd, Cambridge, UK, 2005. [ Links ]
[3] S. R. Yeomans, International Lead Zinc Research Organization, Galvanized steel reinforcement in concrete, Elsevier, Amsterdam (2004). [ Links ]
[4] F. C. Porter, Corrosion Resistance of Zinc and Zinc Alloys, CRC Press, 1994. [ Links ]
[5] B. S. Hamad and J. A. Mike, Constr. Build. Mater., 19, 275-283 (2005). doi:10.1016/j.conbuildmat.2004.07.008. [ Links ]
[6] A. Macias, Mater. Struct., 24, 456-465 (1991). [ Links ]
[7] M. T. Blanco, C. Andrade, A. Macias, Brit. Corros. J., 19, 41-48 (1984). doi:10.1179/000705984798273524. [ Links ]
[8] A. Macias, C. Andrade, Corros. Sci., 30, 393-407 (1990). doi:10.1016/0010- 938X(90)90046-8. [ Links ]
[9] Comité euro-international du béton. Coating Protection for Reinforcement: State of the Art Report, Thomas Telford, ed., London (1995). [ Links ]
[10] Hot-Dip Galvanizing for Corrosion Prevention - A Guide to specifying and Inspecting Hot-Dip Galvanized Reinforcing Steel, American Galvanizers Association, USA (2004).
[11] F. E. Goodwin, T. F. M. Committee, M. Dubois and J .S. Kim (Zinc-based steel coating systems: production and performance ) in Proceedings of International Symposium Held at the TMS Annual Meeting, Minerals, Metals & Materials Society, February, San Antonio, Texas, 16-19 (1998). [ Links ]
[12] M. J. Hornsby, Hot-dip galvanizing: a guide to process selection and galvanizing practice, Intermediate Technology Publications, by arrangement with Food and Agriculture Organisation of the United Nations (1995).
[13] P. Maass, P. Peissker, Handbook of Hot-Dip Galvanization, Jonh Wiley & Sons (2011). [ Links ]
[14] C. J. Slunder, W. K. Boyd, T .K. Christman, International Lead Zinc Research Organization, Zinc: its corrosion resistance, International Lead Zinc Research Organization, New York (1986). [ Links ]
[15] A. C. Stern, Air Pollution, Volume 6: Supplement to Air Pollutants, Their Transformations, Transport, and Effects, 3rd edition, Academic Press, New York (1986). [ Links ]
[16] L. H. Everett, K. W. J. Treadaway, Building Research Station (Great Britain), The use of galvanised steel reinforcement in building, Building Research Station, Ministry of Public Building and Works, Garston, Watford [England] (1970). [ Links ]
[17] G. A. El-Mahdy, A. Nishikata and T. Tsuru, Corros. Sci., 42, 183-194 (2000). doi:10.1016/S0010-938X(99)00057-8. [ Links ]
[18] V. Padilla, P. Ghods and A. Alfantazi, Constr. Build. Mater., 40, 908–918 (2013). doi:10.1016/j.conbuildmat.2012.09.077. [ Links ]
[19] A. P. Yadav, A. Nishikata, T. Tsuru, Corros. Sci., 46, 361–376 (2004). doi:10.1016/S0010-938X(03)00153-7. [ Links ]
[20] D. Zaarei, A. A. Sarabi, F. Sharif and S. M. Kassiriha, J. Coat. Technol. Res., 5, 241–249 (2008). doi:10.1007/s11998-007-9065-5. [ Links ]
[21] A. P. Yadav, A. Nishikata and T. Tsuru, Corros. Sci., 46, 169–181 (2004). doi:10.1016/S0010-938X(03)00130-6. [ Links ]
[22] C. E. Bird, The Influence of Minor Constituents of Portland Cement on the Behaviour of Galvanized Steel in Concrete, South African Council for Scientific and Industrial Research (1964). [ Links ]
[23] W. Lieber, J. Gebauer, Zem.-Kalk-Gips, 4, 161–164 (1969). [ Links ]
[24] G. Rehm, A. Lämmke, Untersuchungen über reaktionen des zinks unter einwirkung von alkalien im Himblick auf das verhalten verzinkter stähle im beton, Betonsteinzeitung, 36, 360–365 (1970). [ Links ]
[25] R. Grauer and H. Kaesche, Corros. Sci., 12, 617–624 (1972). doi:10.1016/ S0010-938X(72)80061-1. [ Links ]
[26] F. Liebau and A. Amel-Zadeh, Krist. Tech., 7, 221.227 (1972). doi:10.1002/ crat.19720070124. [ Links ]
[27] A. M. S. El Din, F. M. Abd El Wahab, S. M. Abd El Haleem, Mater. Corros., 24, 389–394 (1973). doi:10.1002/maco.19730240508. [ Links ]
[28] L. Vorkapic, D. M. Draic, and A. R. Despic, J. Electrochem. Soc., 121, 1385–1392 (1974). [ Links ]
[29] R. Duval, R. Arliaguie, Mem. Sci. Rev. Met., 71, 719-727 (1974). [ Links ]
[30] Z. Zembura, L. Burzynska, Corros. Sci., 17, 871–878 (1977). doi:10.1016/0010-938X(77)90093-2. [ Links ]
[31] L. M. Baugh and A. Higginson, Electrochim. Acta, 30, 1163–1172 (1985). doi:10.1016/0013-4686(95)80008-5. [ Links ]
[32] A. Macias and C. Andrade, Brit. Corros. J., 22, 113-118 (1987). doi:10.1179/000705987798271631. [ Links ]
[33] A. Macias and C. Andrade, Brit. Corros. J., 22, 119–130 (1987). doi:10.1179/000705987798271749. [ Links ]
[34] A. Macías, C. Andrade, Mater. Construcc., 36, 19-27 (1986) . [ Links ]
[35] C. Andrade, (Reinforcement corrosion: Research needs) in Concrete Repair Rehabilitation and Retrofitting II, Taylor & Francis Group, London, UK (2009). [ Links ]
[36] A. Macías and C. Andrade, Cement Concrete Res., 17, 307-316 (1987). doi:10.1016/0008-8846(87)90113-X. [ Links ]
[37] A. Macías and C. Andrade, Brit. Corros. J., 18, 82-87 (1983). [ Links ]
[38] M. C. Andrade, A. Macias (Galvanized Reinforcements in Concrete), in Surface Coatings2 (A. D. Wilson, J. W. Nicholson, H. J. Prosser, Eds.), Springer Netherlands, pp. 137-182 (1988). [ Links ]
[39] T. Bellezze, M. Malavolta, A. Quaranta, N. Ruffini and G. Roventi, Cement Concrete Comp., 28, 246-255 (2006). doi: 10.1016/jcemconcomp. 2006.01.011. [ Links ]
[40] A. S. Castela, B. S. da Fonseca, R. G. Duarte, R. Neves and M. F.Montemor, Electrochim. Acta, 124, 52-68 (2014). doi:10.1016/j.electacta.2013.11.157. [ Links ]
[41] S. B. Farina and G .S. Duffó, Electrochim. Acta, 52, 5131–5139 (2007). doi:10.1016/j.electacta.2007.01.014. [ Links ]
[42] I. Fayala, L. Dhouibi, X. R. Nóvoa and M. Ben Ouezdou, Cement Concrete Comp., 35, 181-189 (2013). doi:10.1016/j.cemconcomp.2012.08.014. [ Links ]
[43] R. Ghosh and D. D. N. Singh, Surf. Coat. Tech., 201, 7346–7359 (2007). doi:10.1016/j.surfcoat.2007.01.048. [ Links ]
[44] M. Raupach, and Institute of Materials, Minerals and Mining, Corrosion of reinforcement in concrete: mechanisms, monitoring, inhibitors and rehabilitation techniques, Woodhead, CRC Press, Cambridge, Boca Raton (2007). [ Links ]
[45] E. Sistonen (Service life of hot-dip galvanised reinforcement bars in carbonated and chloride-contaminated concrete), dissertation for the degree of Doctor of Science in Technology, Faculty of Engineering and Architecture, Helsinki University of Technology, December, Finland (2009). [ Links ]
[46] Z. Q. Tan and C. M. Hansson, Corros. Sci., 50, 2512-2522 (2008). doi:10.1016/j.corsci.2008.06.035. [ Links ]
[47] F. Tittarelli and G. Moriconi, Cement Concrete Res., 41, 609–614 (2011). doi:10.1016/j.cemconres.2011.03.011. [ Links ]
[48] E. Sistonen, A. Cwirzen and J. Puttonen, Corros. Sci., 50, 3416–3428 (2008). doi:10.1016/j.corsci.2008.08.050. [ Links ]
[49] R. Roberge and W. Zheng, Corros. Sci., 35, 507–514 (1993). doi:10.1016/0010-938X(93)90183-H. [ Links ]
[50] E. Maahn and B. Sorensen, Corrosion, 42, 187-196 (1986). doi:10.5006/1.3585996. [ Links ]
[51] N. R. Short, S. Zhou and J. K. Dennis, Surf. Coat. Tech., 79, 218-224 (1996). doi:10.1016/0257-8972(95)02428-X. [ Links ]
[52] M. Heydarzadeh Sohi and M. Jalali, J. Mater. Process. Tech., 138, 63-66 (2003). doi:10.1016/S0924-0136(03)00050-5. [ Links ]
[53] A. K. Singh, G. Jha, N. Rani, N. Bandyopadhyay and T. Venugopalan, Surf. Coat. Tech., 200, 4897–4903 (2006). doi:10.1016/j.surfcoat.2005.04.044. [ Links ]
[54] M. Kulka and A. Pertek, Appl. Surf. Sci., 218, 114-123 (2003). doi:10.1016/ S0169-4332(03)00563-4. [ Links ]
[55] M. A. Arenas, C. Casado and J. D. Damborenea, Cement Concrete Comp., 28, 267-275 (2007). doi:10.1016/j.cemconcomp.2006.01.010. [ Links ]
[56] S. M. A. Shibli and F. Chacko, Surf. Coat. Tech., 202, 4971–4975 (2008). doi:10.1016/j.surfcoat.2008.04.090. [ Links ]
[57] H. Asgari, M. R. Toroghinejad and M. A. Golozar, Curr. Appl. Phys., 9,59–66. (2009). doi:10.1016/j.cap.2007.10.090. [ Links ]
[58] Y. Boonyongmaneerat, K. Saengkiettiyut, P. Rattanawaleedirojn, C. Angkaprasert, J. Wanichsampan and S. Saenapitak, J. Iron Steel Res. Int., 17, 74-78 (2010). doi:10.1016/S1006-706X(10)60132-X. [ Links ]
[59] Z. A. Hamid, A. A. Aal, H. B. Hassan and A. Shaaban, Appl. Surf. Sci., 256, 4166-4170 (2010). doi:10.1016/j.apsusc.2010.01.119. [ Links ]
[60] S. Le Manchet, D. Verchère and J. Landoulsi, Thin Solid Films, 520, 2009- 2016 (2012). doi:10.1016/j.tsf.2011.09.064. [ Links ]
[61] E. Pavlidou, N. Pistofidis, G. Vourlias and G. Stergioudis, Mater. Lett., 59, 1619–1622 (2005). doi:10.1016/j.matlet.2004.08.045. [ Links ]
[62] R. Sa-nguanmoo, E. Nisaratanaporn and Y. Boonyongmaneerat, Corros. Sci., 53, 122-126 (2011). doi:10.1016/j.corsci.2010.09.031. [ Links ]
[63] X.-L. Shang, B. Zhang, E.-H. Han and W. Ke, Electrochim. Acta, 65, 294- 304 (2012). doi:10.1016/j.electacta.2012.01.078. [ Links ]
[64] S. M. A. Shibli, R. Manu and S. Beegum, Surf. Coat. Tech., 202, 1733-1737 (2008). doi:10.1016/j.surfcoat.2007.07.033. [ Links ]
[65] S. T. Vagge and V. S. Raja, Surf. Coat. Tech., 203, 3092–3098 (2009). doi:10.1016/j.surfcoat.2009.03.026. [ Links ]
[66] D. Yang, J. Chen, Q. Han and K. Liu, J. Rare Earths, 27, 114-118 (2009). doi:10.1016/S1002-0721(08)60203-3. [ Links ]
[67] J. D. Culcasi, C. I. Elsner, A. R. D. Sarli and P. R. Sere, Surf. Coat. Tech., 122, 143-149 (1999). [ Links ]
[68] N. Pistofidis, G. Vourlias, S. Konidaris, E. Pavlidou, A. Stergiou and G. Stergioudis, Mater. Lett. 61, 994–997 (2007). doi:10.1016/j.matlet.2006.06.029. [ Links ]
[69] J. H. Park, W. S. Kim, D.-H. Jo, J. S. Kim and J. M. Park, J. Ind. Eng. Chem., 20, 1965- 1972 (2014). doi:10.1016/j.jiec.2013.09.018. [ Links ]
[70] S. M. A. Shibli, V. S. Dilimon, S. P. Antony and R. Manu, Surf. Coat. Tech., 200, 4791–4796 (2006). doi:10.1016/j.surfcoat.2005.04.058. [ Links ]
[71] F. J. Recio, M. C. Alonso, L. Gaillet and M. Sánchez, Corros. Sci., 53, 2853– 2860 (2011). doi:10.1016/j.corsci.2011.05.023. [ Links ]
[72] A. A. O. Magalhães, I. C. P. Margarit and O. R. Mattos, Electrochim. Acta, 44, 4281–4287 (1999). doi:10.1016/S0013-4686(99)00143-7. [ Links ]
[73] R. B. Figueira, C. J. Silva, E. V. Pereira, M. M. Salta, J. Electrochem. Soc., 160, C467–C479 (2013). [ Links ]
[74] D. Liu, Z. Yang, Z. Wang and C. Zhang, Surf. Coat. Tech., 205, 2328–2334 (2010). doi:10.1016/j.surfcoat.2010.09.018. [ Links ]
[75] F. Tittarelli and G. Moriconi, Corros. Sci., 52, 2958–2963 (2010). doi:10.1016/j.corsci.2010.05.008. [ Links ]
[76] S. González, M. Gil, J. Hernández, V. Fox and R. Souto, Prog. Org. Coat.,41, 167–170 (2001). doi:10.1016/S0300-9440(01)00139-4. [ Links ]
[77] M. F. Montemor, A .M. Simões and M. G. S. Ferreira, Composition and behaviour of cerium films on galvanised steel, Prog. Org. Coat., 43, 274–281. (2001). [ Links ]
[78] K. Aramaki, Corros. Sci., 44, 1375–1389 (2002). doi:10.1016/S0010- 938X(01)00138-X. [ Links ]
[79] M. Hara, R. Ichino, M. Okido and N. Wada, Surf. Coat. Tech., 169–170, 679-681 (2003). doi:10.1016/S0257-8972(03)00064-1. [ Links ]
[80] I. M. Zin, S. B. Lyon and V. I. Pokhmurskii, Corros. Sci., 45, 777–788 (2003). [ Links ]
[81] M. Garcia-Heras, A. Jimenez-Morales, B. Casal, J .C. Galvan, S. Radzki and M. A. Villegas, J. Alloys Compd., 380, 219–224 (2004). doi:10.1016/j. jallcom.2004.03.047. [ Links ]
[82] T. Prosek and D. Thierry, Prog. Org. Coat., 49, 209–217 (2004). doi:10.1016/j.porgcoat.2003.09.012. [ Links ]
[83] S. Feliu and V. Barranco, Characterization of a Lacquer Film Formulated with Phosphating Reagents for Corrosion Protection of Galvanized Substrates, JCT Research, 1, 93–102 (2004) [ Links ]
[84] M. G. Ferreira, R. Duarte, M. F. Montemor and A. M. Simões, Electrochim. Acta, 49, 2927–2935 (2004). doi:10.1016/j.electacta.2004.01.051. [ Links ]
[85] A. A. O. Magalhães, I. C. P. Margarit and O. R. Mattos, J. Electroanal. Chem., 572, 433–440 (2004). doi:10.1016/j.jelechem.2004.07.016. [ Links ]
[86] C. G. da Silva, A. N. Correia, P. de Lima-Neto, I. C. P. Margarit and O. R. Mattos, Corros. Sci., 47, 709–722 (2005). doi:10.1016/j.corsci.2004.07.008. [ Links ]
[87] S. Dalbin, G. Maurin, R. P. Nogueira, J. Persello and N. Pommier, Surf. Coat. Tech.,194, 363–371 (2005). doi:10.1016/j.surfcoat.2004.07.126. [ Links ]
[88] W. Trabelsi, P. Cecilio, M. G. S. Ferreira and M. F. Montemor, Prog. Org. Coat., 54, 276–284. (2005). doi:10.1016/j.porgcoat.2005.07.006. [ Links ]
[89] M. Eliziane, P. D. Souza, E. Ariza, M. Ballester, I. Valéria and P. Yoshida, Mater. Res., 9, 59–64 (2006). [ Links ]
[90] A. Conde, J. Damborenea, A. Durán and M. Menning, J. Sol-Gel Sci. Techn., 37, 79–85. (2006). doi:10.1007/s10971-005-5357-3. [ Links ]
[91] M. Sánchez, M. C. Alonso, P. Cecílio, M. F. Montemor and C. Andrade, Cement Concrete Comp., 28, 256–266 (2006). doi:10.1016/j. cemconcomp.2006.01.004. [ Links ]
[92] R. Romero Pareja, R. López Ibáñez, F. Martín, J. R. Ramos-Barrado and D. Leinen, Surf. Coat. Tech., 200, 6606–6610 (2006). doi:10.1016/j. surfcoat.2005.11.098. [ Links ]
[93] J. Lu, H. Wu, G. Kong, C. Che and Q. Xu, Trans. Nonferrous Met. Soc. China, 16, 1397–1401 (2006). doi:10.1016/S1003-6326(07)60027-2. [ Links ]
[94] A. M. Cabral, W. Trabelsi, R. Serra, M. F. Montemor, M. L. Zheludkevich and M. G. S. Ferreira, Corros. Sci., 48, 3740–3758 (2006). doi:10.1016/j. corsci.2006.01.010. [ Links ]
[95] M. F. Montemor, W. Trabelsi, M. Zheludevich and M. G. S. Ferreira, Prog. Org. Coat., 57, 67-77 (2006). doi:10.1016/j.porgcoat.2006.06.009. [ Links ]
[96] M. F. Montemor, A. M. Cabral, M. L. Zheludkevich and M. G. S. Ferreira, Surf. Coat. Tech., 200, 2875–2885 (2006). doi:10.1016/j. surfcoat.2004.11.012. [ Links ]
[97] J. B. Bajat, V. B. Mikovic-Stankovic, N. Bibic and D.M. Draic, Prog. Org. Coat., 58, 323-330 (2007). doi:10.1016/j.porgcoat.2007.01.011. [ Links ]
[98] U. Bexell and T. M. Grehk, Surf. Coat. Tech., 201, 4734–4742. (2007). doi:10.1016/j.surfcoat.2006.10.014. [ Links ]
[99] M. Hosseini, H. Ashassi-Sorkhabi and H. A. Y. Ghiasvand, J. Rare Earths, 25, 537-543 (2007). doi:10.1016/S1002-0721(07)60558-4. [ Links ]
[100] L. Zhu, F. Yang and N. Ding, Surf. Coat. Tech., 201, 7829–7834 (2007). doi:10.1016/j.surfcoat.2007.03.024. [ Links ]
[101] M. F. Montemor and M. G. S. Ferreira, Electrochim. Acta, 52, 6976–6987 (2007). doi:10.1016/j.electacta.2007.05.022. [ Links ]
[102] F. J. Shan, C. S. Liu, S. H. Wang and G. C. Qi, Acta Metall. Sin. Engl. Lett., 21, 245–252 (2008). doi:10.1016/S1006-7191(08)60045-9. [ Links ]
[103] J. B. Bajat, V. B. Mikovic-Stankovic, J. P. Popic and D. M. Draic, Prog. Org. Coat., 63, 201–208 (2008). doi:10.1016/j.porgcoat.2008.06.002. [ Links ]
[104] M. F. Montemor et al., Electrochim. Acta, 53, 5913-5922 (2008). [ Links ]
[105] D. D. N. Singh and R. Ghosh, Surf. Coat. Tech., 202, 4687–4701 (2008). doi:10.1016/j.surfcoat.2008.03.038. [ Links ]
[106] M. Fedel, M. Olivier, M. Poelman, F. Deflorian, S. Rossi and M.- E. Druart, Prog. Org. Coat., 66, 118–128 (2009). doi:10.1016/j. porgcoat.2009.06.011. [ Links ]
[107] G. Kong, J. Lu and H. Wu, J. Rare Earths, 27, 164–168 (2009). doi:10.1016/S1002-0721(08)60213-6. [ Links ]
[108] Y. Hamlaoui, L. Tifouti and F. Pedraza, Corros. Sci., 51, 2455–2462 (2009). doi:10.1016/j.corsci.2009.06.037. [ Links ]
[109] T. Peng and R. Man, J. Rare Earths, 27, 159–163 (2009). doi:10.1016/ S1002-0721(08)60212-4. [ Links ]
[110] F. Deflorian, S. Rossi, M. Fedel and C. Motte, Prog. Org. Coat., 69, 158- 166 (2010). doi:10.1016/j.porgcoat.2010.04.007. [ Links ]
[111] M. Fedel, M.-E. Druart, M. Olivier, M. Poelman, F. Deflorian and S. Rossi, Prog. Org. Coat., 69, 118–125 (2010). doi:10.1016/j. porgcoat.2010.04.003. [ Links ]
[112] G. Kong, J. Lu, S. Zhang, C. Che and H. Wu, Surf. Coat. Tech., 205, 545– 550 (2010). doi:10.1016/j.surfcoat.2010.07.033. [ Links ]
[113] S. Le Manchet, J. Landoulsi, C. Richard and D. Verchère, Surf. Coat. Tech., 205, 475–482 (2010). doi:10.1016/j.surfcoat.2010.07.009. [ Links ]
[114] B. Ramezanzadeh, M. M. Attar and M. Farzam, Surf. Coat. Tech., 205, 874–884 (2010). doi:10.1016/j.surfcoat.2010.08.028. [ Links ]
[115] M. Olivier, A. Lanzutti, C. Motte and L. Fedrizzi, Corros. Sci., 52, 1428– 1439 (2010) doi:10.1016/j.corsci.2010.01.011. [ Links ]
[116] G. Kong, L. Liu, J. Lu, C. Che and Z. Zhong, J. Rare Earths, 28, 461–465 (2010). doi:10.1016/S1002-0721(09)60134-4. [ Links ]
[117] R. Romero, F. Martin, J. R. Ramos-Barrado and D. Leinen, Surf. Coat. Tech., 204, 2060–2063 (2010). doi:10.1016/j.surfcoat.2009.10.006. [ Links ]
[118] A. K. Guin, S. K. Nayak, T. K. Rout, N. Bandyopadhyay and D. K. Sengupta, J. Coat. Technol. Res., 9, 97–106 (2011). doi:10.1007/s11998-011-9321-6. [ Links ]
[119] E. Huttunen-Saarivirta, V. E. Yudin, L. A. Myagkova and V. M. Svetlichnyi, Prog. Org. Coat., 72, 269–278. (2011). doi:10.1016/j. porgcoat.2011.04.015. [ Links ]
[120] S. Jafarzadeh, A. Adhikari, P.-E. Sundall and J. Pan, Prog. Org. Coat., 70, 108–115 (2011). doi:10.1016/j.porgcoat.2010.10.011. [ Links ]
[121] A. M. P. Simões, R. O. Carbonari, A. R. D. Sarli, B. del Amo and R. Romagnoli, Corros. Sci., 53, 464–472. (2011). doi:10.1016/j.corsci.2010.09.058. [ Links ]
[122] G. Kong, L. Lingyan, J. Lu, C. Che and Z. Zhong, Corros. Sci., 53, 1621- 1626 (2011). doi:10.1016/j.corsci.2011.01.038. [ Links ]
[123] B. Ramezanzadeh and M. M. Attar, Prog. Org. Coat., 71, 314–328 (2011). doi:10.1016/j.porgcoat.2011.03.026. [ Links ]
[124] Z. Zou, N. Li, D. Li, H. Liu and S. Mu, J. Alloys Compd., 509, 503–507 (2011). doi:10.1016/j.jallcom.2010.09.080. [ Links ]
[125] M. Yuan, J. Lu, G. Kong and C. Che, Surf. Coat. Tech., 205, 4507–4513 (2011). doi:10.1016/j.surfcoat.2011.03.088. [ Links ]
[126] C. Motte, M. Poelman, A. Roobroeck, M. Fedel, F. Deflorian and M.-G. Olivier, Prog. Org. Coat., 74, 326–333 (2012). doi:10.1016/j. porgcoat.2011.12.001. [ Links ]
[127] I. A. Kartsonakis, A. C. Balaskas, E. P. Koumoulos, C. A. Charitidis and G. C. Kordas, Corros. Sci., 57, 30-41 (2012). doi:10.1016/j. corsci.2011.12.037. [ Links ]
[128] J. Min, J. H. Park, H.-K. Sohn and J. M. Park, J. Ind. Eng. Chem., 18, 655- 660 (2012). doi:10.1016/j.jiec.2011.11.057. [ Links ]
[129] L. Wang, C. Liu, H. Yu and C. An, J. Iron Steel Res. Int., 19, 46-51 (2012). doi:10.1016/S1006-706X(13)60019-9. [ Links ]
[130] M. F. Montemor, D. V. Snihirova, M. G. Taryba, S. V. Lamaka, I. A. Kartsonakis, A. C. Balaskas, et al., Electrochim. Acta, 60, 31-40 (2012). doi:10.1016/j.electacta.2011.10.078. [ Links ]
[131] M. Fedel, E. Callone, S. Diré, F. Deflorian, M.-G. Olivier and M. Poelman, Electrochim. Acta, 124, 90-99 (2014). doi:10.1016/j. electacta.2013.11.006. [ Links ]
[132] R. Z. Zand, K. Verbeken and A. Adriaens, Influence of the Cerium Concentration on the Corrosion Performance of Ce-doped Silica Hybrid Coatings on Hot Dip Galvanized Steel Substrates, Int. J. Electrochem. Sci., 8 PAG. XX (2013). [ Links ]
[133] D. Xue and W. J. Van Ooij, Prog. Org. Coat., 76, 1095–1102 (2013). doi:10.1016/j.porgcoat.2013.03.004. [ Links ]
[134] R. Sako and J. Sakai, Surf. Coat. Tech., 219, 42-49 (2013). doi:10.1016/j. surfcoat.2012.12.050. [ Links ]
[135] C. M. Hansson, A. Poursaee and S. J. Jaffer (Corrosion of reinforcing bars in concrete), Portland Cement Association, Skokie, USA (2007). [ Links ]
Artigo submetido em Junho de 2014 e aceite em Setembro de 2014













