Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Corrosão e Protecção de Materiais
versão On-line ISSN 2182-6587
Corros. Prot. Mater. vol.32 no.2 Lisboa jun. 2013
ARTIGO
Ni-co alloys electrodeposited in stainless steel for supercapacitors electrodes#
Ligas ni-co eletrodepositadas em aço inoxidável para elétrodos de supercondensadores
R. P. Silva(*),(1), S. Eugénio(1), T. M. Silva(1),(3), M. J. Carmezim(1),(2) and M. F. Montemor(1),(4)
(1) ICEMS, Instituto Superior Técnico, TULisbon, Av. Rovisco Pais, 1, 1049 – 001 Lisboa, Portugal.
(2) Instituto Politécnico de Setúbal, ESTSetubal, Campus IPS, 2910 Setúbal, Portugal.
(3) Department of Mechanical Engineering, Instituto Superior de Engenharia de Lisboa, 1959-007 Lisboa, Portugal.
(4) Department of Chemical Engineering, Instituto Superior Técnico, TULisbon, Av. Rovisco Pais, 1, 1049-001 Lisboa, Portugal.
(*)Corresponding author, e-mail: rui.pedro.silva@ist.utl.pt
ABSTRACT
The development of electric energy storage devices with high power density has become one of the most challenging research areas in a wide range of applications. In this context, electrochemical supercapacitors are attracting great attention due to their high potential density, fast charge-discharge cycles and high number of life cycles.
In the present work, Ni-Co nanostructured films were produced by electrodeposition technique in order to form a porous dendritic structure with enhanced surface area.
The electrochemical behaviour of the deposited films was studied by charge-discharge experiments. The Ni-Co dendritic films electrodeposited at current densities i1 = -4 mA cm-2 and i2 = -30 mA cm-2 display a specific capacitance of 215 F g-1. The capacitance loss is about 33 % after 6000 charge-discharge cycles.
Keywords: Supercapacitors, Electrodeposition, Ni-Co Alloys, Specific Capacitance, Dendritic Structure
RESUMO
O desenvolvimento de dispositivos de armazenagem de energia com elevada densidade de potência tornou-se numa área de investigação de grande interesse para um vasto campo de aplicações. Os supercondensadores eletroquímicos para além de elevada densidade de potência possuem tempos de carga e descarga reduzidos e elevado número de ciclos de vida. No presente trabalho, produziram-se filmes de Ni-Co nanoestruturados por eletrodeposição com uma estrutura dendrítica de elevada área superficial.
O comportamento eletroquímico dos filmes depositados foi estudado através de ensaios de carga-descarga. Os filmes depositados a densidades de corrente i1= -4 mA cm-2 e i2= -30 mA cm-2 apresentaram um valor de capacidade específica de 215 F g-1. A perda de capacidade é de 33 % ao fim de 6000 ciclos de carga descarga.
Palavras-chave: Supercondensadores, Eletrodeposição, Ligas Ni-Co, Capacidade Específica, Estrutura Dendrítica
1. INTRODUCTION
The demand for more efficient, sustainable and environmental friendly transports as well as electronic portable devices with high autonomy has stimulated the development of energy storage systems as batteries and supercapacitors. Supercapacitors are considered the direction of future commercialization and applications due to their ability to stand a very large number of charge and discharge cycles, increased power density and increased lifetime with relatively low maintenance costs. However, despite the current research and development achievements, electrochemical supercapacitors still lack improved performance in terms of energy density. In order to overcome this drawback it is of paramount importance to develop new electrode materials with optimal properties [1].
Extensive research has been performed on improvements of supercapacitors performances [2-4]. Supercapacitors electrodes demand a combination of several characteristics, such as: high conductivity, high surface area, corrosion resistance, temperature stability, controlled pore structure and low cost. Recent trends on the use of metallic oxides for supercapacitors show that transition metal oxides, possessing multiple oxidation states, which enable reversible redox reactions for pseudocapacitance generation, are desirable [5].
Among these, RuO2 shows very high specific capacitances (Sc), which result from faradaic processes involving the transition between various oxidation states of Ru ions. These redox processes are accompanied by proton intercalation in order to preserve the electroneutrality of the oxide [6]. Other oxides of transition metals such as Co3O4, NiO, SnO2, MnO2, PbO2 and V2O5 are being investigated as electrodes for supercapacitors in aqueous electrolytes. Recently, Ni-Co based systems have been proposed as materials for redox electrochemical supercapacitors electrodes due to their multiple oxidation states and high theoretical specific capacitances [4, 7]. Co3O4 is well known by its excellent reversible redox behavior, high conductivity, long term performance and good corrosion stability [4]. The pseudocapacitance of this oxide originates from the following redox reaction [8]:
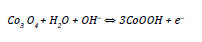
Co(OH)2 is also an attractive material due to its layered structure and large interlayer spacing, which warrants a high surface area and fast ion insertion/desertion rate that occurs during the following reaction [4, 8]:
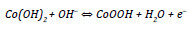
Nickel oxide is considered an alternative electrode material for supercapacitors due to its easy synthesis, relatively high specific capacitance, environment friendliness, and low cost [4]. The redox reaction of nickel oxide in KOH electrolyte can be expressed as [9]:
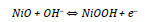
The challenges in using NiO based electrode materials involve poor cycle performance and high resistivity. The conductivity of nickel oxide can be improved by introducing cobalt ions into the nickel oxide matrix [4]. Nickel-cobalt binary metal oxides such as nickel cobaltite (NiCo2O4), have shown increased conductivity and improved electrochemical activity when compared to their single oxides due to the combined contributions from both nickel and cobalt ions in the oxide structure [10].
On the other hand, porous 3D structures act as conductive networks and enable the access of ions and electrons to the active sites on the surface, resulting in a better electrochemical response of the electrode. These structures include, among others, NiO nanorods [11], Ni(OH)2 nano-flakes formed on carbon nanotubes and nickel foams [12], all of them presenting very good results. In this context, the properties of dendritic structures have been less explored. However, 3D dendritic structures fabricated, for example, using the H2 bubble template [13] are promising candidates for supercapacitor electrodes due to their high surface area and porosity.
In the present work, electrodeposition of Ni-Co films with a 3D dendritic structure was performed on stainless steel substrates from chloride-based electrolytes. Stainless steel substrates are cheap, mechanically stable and corrosion resistant, making them very attractive for electrode fabrication. Using a square wave-form in the cathodic domain and applying different current densities, the film morphology was tailored in order to develop the 3D branched dendritic structures with a large open porosity, supported in a continuous compact layer. Thus, with this innovative approach it was possible to fabricate a new 3D dendritic Ni-Co film structure on stainless steel electrodes using current densities one order of magnitude lower than those typically found in the literature [14, 15]. This strategy opens a new array of technical possibilities for fabrication of supercapacitor electrodes using cheaper and reliable routes. The work highlights the effect of the applied current on the mechanism of formation of the 3D dendritic structures and gives relevant insights concerning the composition and morphology of these dendritic structures with ability to be used as electrodes for redox supercapacitors.
2. EXPERIMENTAL PROCEDURES
Electrodeposition
Stainless steel substrates (AISI 304 from Goodfellow) were polished with 500, 800, and 1000 grit SiC abrasive paper, thoroughly rinsed with distilled water, and dried with jet of compressed air. The Ni-Co alloy was electrodeposited on these substrates from an aqueous electrolyte containing 0.07 M CoCl2.6H2O, 0.03 M NiCl2.6H2O and 0.5 M H3BO3. The electrolyte pH value was set at 5.5 by addition of NaOH.
Electrodeposition was performed in a conventional three-electrode electrochemical cell, using a platinum (Pt) plate as counter electrode and a saturated calomel electrode (SCE) as reference. The process was carried at room temperature.
A Voltalab PGZ 100 potentiostat from Radiometer was used for the simultaneous electrodeposition of cobalt and nickel by imposing a square-wave cathodic current. The upper current limit (i1) was varied from -3 to -5 mA cm-2 and the lower current limit (i2) was varied from -5 to -50 mA cm-2. The pulse duration was the same for both current limits (50 s) and a total of 20 cycles was applied in each electrodeposition experiment.
Characterization
The microstructure and chemical composition of the deposited Ni-Co films were analyzed by scanning electron microscopy (SEM, Hitachi S2400) and energy dispersive X-ray spectroscopy (EDS, Rontec standard detector), respectively.
The electrochemical characterization of Ni-Co alloys was performed by charge-discharge curves in 1 M KOH electrolyte with current density of 1 mA cm-2. The electrochemical characterization has been acquired in the electrochemical cell configuration described above, using the electrodeposited Ni-Co alloy as working electrode.
3. RESULTS AND DISCUSSION
The dependence of the electrodeposited mass, measured experimentally, with the lower (more negative) pulse limit current (i2) is depicted in Figure 1. For comparison purposes, the theoretical mass, calculated from Faraday´s law, is also shown. This calculation was performed assuming the average molar mass of Ni and Co and the total charge of electrodeposition. It is observed that the electrodeposited mass increases proportionally with the modulus of i2. Furthermore, for i2 = -10 and -15 mA cm-2, the deposited mass is very close to the theoretical value. Assuming that the current efficiency for the electrodeposition process is given by the ratio between the experimental and theoretical values, in that current range the efficiency of the Ni-Co electrodeposition is ca. 97 %. When more negative i2 values are applied, the deposited mass starts to deviate from its theoretical value, and a decreased in the deposition current efficiency is observed, being approximately 83 % for i2 = -40 mA cm2. This deviation can be explained by more intense H2 evolution, occurring simultaneously with Ni-Co deposition [16].
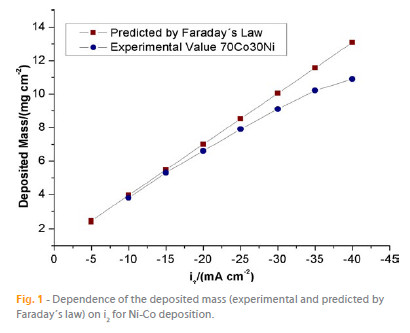
The chemical composition of the electrodeposited Ni-Co films was determined by EDS, and its dependence on i2 is shown in Figure 1. As it can be observed, for i2 values more positive than -15 mA cm-2, the atomic percentage of Co in the electrodeposited Ni-Co alloy is higher than that in deposition electrolyte. Hence, in this current range, the Ni-Co deposition reveals its typical anomalous behavior with the less noble metal (Co) being preferentially deposited [17]. This anomalous deposition is often found in the iron-group binary alloys [17] and, although this phenomenon has been extensively reviewed in the literature, it is still not fully understood [17, 18]. When the applied i2 is in the range -15 to -30 mA cm-2, the atomic percentage of Co and Ni in the deposit approaches that of the electrolyte (Figure 2), which indicates an equilibrium deposition behavior. This effect is in accordance with literature data reported for galvanostatic deposition of Ni-Co alloys [17]. In conclusion, the results obtained in this work show that the Co content decreases with increasing current density and, at sufficiently high current densities (more than -20 mA cm-2) the metal ratio of the deposit approaches that of the electrolyte.
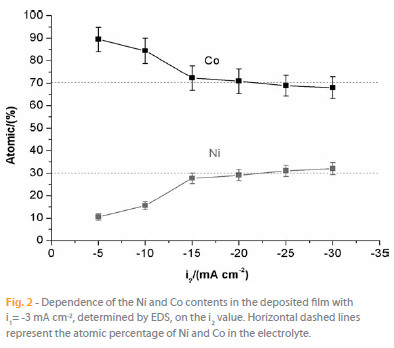
Figure 3 depicts SEM images of Ni-Co films deposited at different i2 values. It can be observed that there is a clear change in growth type regime as the i2 current values become more negative than -15 mA cm-2, when a 3D dendritic morphology starts to nucleate. In fact, for current densities of -5 and -10 mA cm-2 (Figure 3a, 3b), the electrodeposited Ni-Co films are smoother, consisting of wellorganised micrometric grains, whose size increases with decreasing i2. For i2 = -15 mA cm-2 (Figure 3c), most of the Ni-Co film presents an open porous structure, revealing the formation of dendrites without significant branching. However, in some locations, more developed dendrites can also be observed. For i2 = -20 mA cm-2 (Figure 3d), the density of fully developed dendrites, with relevant secondary branching, increases as well as their sizes (diameter and height). Each dendrite consists of several hierarchical assemblies, which develop from a central longitudinal core, to which several secondary branches with different lengths and widths are connected.
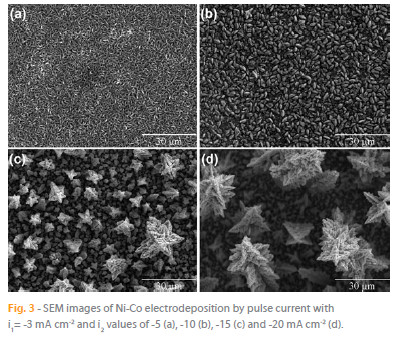
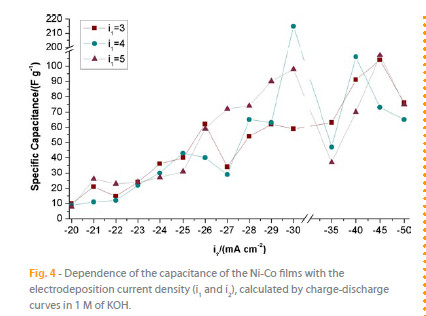
Figure 4 depicts the capacitance evolution with the current density (i1 and i2) applied in electrodeposition process. The capacitance values were calculated by charge-discharge curves of the Ni-Co electrode in 1M KOH electrolyte at a galvanostatic current density of 1 mA cm-2 in a potential range of 0 to 0.4 V. An unstable increase of the capacitance value is observed until -45 mA cm-2 due to the growing of specific surface area. The maximum value of capacitance (215 F g-1) was obtained for i1 = -4 e i2 = -30 mA cm-2. Comparing with the literature [5, 10, 11, 19] this value is in the range of capacitance values for transition metals electrodes (50 and 1100 F g-1) but still far from the top values.
The effect of KOH concentration in the electrolyte on the chargedischarge curves of Ni-Co film is represented in Figure 5. It is observed that a higher concentration of KOH leads to a longer total time for charge-discharge which means a higher value of capacitance. This effect can be explained by looking at the redox equations occurring at the Ni-Co film. Both Co and Ni oxidation reactions are depicted in the following equations [8, 9]:
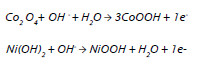
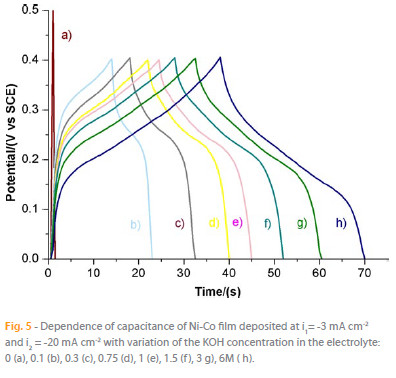
According to the equations, OH- is essential for oxidation reactions of both Ni and Co justifying the increase of capacitance with KOH concentration.
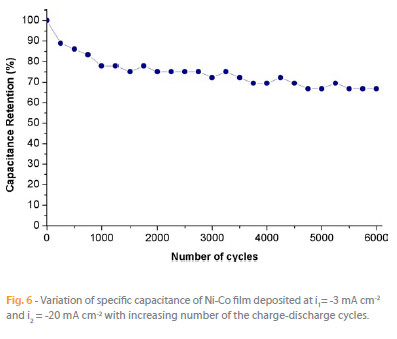
Figure 6 shows the dependence of the discharge specific capacitance of the Ni-Co films on the charge-discharge cycles numbers. The charge- -discharge experiments were performed at current density of 1 mA cm-2 within the potential window of 0 to 0.4 V in 1M KOH electrolyte. The discharge specific capacitance decreased to ca. 70 % after 1500 cycles and then remains constant up to 6000 cycles, indicating that Ni-Co electrode has relatively good electrochemical stability. The repetitive charge-discharge cycling does not induce significant changes in the dendritic structures.
4. CONCLUSIONS
Ni-Co nanostrutured films are established as good candidates for new electrochemical supercapacitor materials. A new approach consisting on applying a square current waveform in the cathodic domain was successfully applied for the electrodeposition of Ni-Co films. This approach allows tailoring the properties of the deposited Ni-Co films (morphology, porosity, chemical composition) through the control of the electrodeposition parameters. This method has proved to be effective to fabricate a continuous film over which 3D Ni-Co dendrites with highly ramified branches and open porous structures were grown.
When the lower limit pulse is less negative, well-organized grain morphologies were observed for Ni-Co film; the composition follows the anomalous deposition and the deposited mass is in accordance with the Faraday law. On the other hand, for more negative values of the lower limit pulse, there is the formation of a 3D porous network dendritic structure with a high superficial area and the Ni-Co ratio approaches that of the electrolyte.
The electrochemical tests confirm that nanostructures electrodeposited with i1 = -4 and i2 = -30 mA cm-2 show the highest capacitance (215 F g-1). Almost 70 % of specific capacitance remained after 6000 cycles. Detailed studies on the electrochemical behavior of the present materials are under investigation to enhance their performance.
REFERENCES
[1] X. Zhao, B. M. Sanchez, P. J. Dobson and P. S. Grant, Nanoscale, 3, 3, 839 (2011). [ Links ]
[2] M. Jayalakshmi and K. Balasubramanian, Int. J. Electrochem. Sci., 3, 1196 (2008). [ Links ]
[3] C. Liu, F. Li, L.-P. Ma and H.-M. Cheng, Adv. Mater., 22, 8, E28 (2010). [ Links ]
[4] G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 41, 2, 797 (2012). [ Links ]
[5] C. D. Lokhande, D. P. Dubal and O.-S. Joo, Current Appl. Phys., 11, 3, 255 (2011). [ Links ]
[6] J. P. Zheng, P. J. Cygan and T. R. Jow, J. Electrochem. Soc., 142, 2699, (1995). [ Links ]
[7] Y. Zhang, H. Feng, X. Wu, L. Wang, A. Zhang, T. Xia, H. Dong, X. Li and L. Zhang, Int. J. Hydrogen Energy, 34, 11, 4889 (2009). [ Links ]
[8] I. G. Casella and M. Gatta, J. Electroanal. Chem., 534, 1, 31 (2002). [ Links ]
[9] S.-L. Yau, F.-R. F. Fan, T. P. Moffat and A. J. Bard, J. Phys. Chem., 98, 21, 5493 (1994). [ Links ]
[10] R. R. Salunkhe, K. Jang, H. Yu, S. Yu, T. Ganesh, S.-H. Han and H. Ahn, J. Alloys Compd., 509, 23, 6677 (2011). [ Links ]
[11] Z. Lu, Z. Chang, J. Liu and X. Sun, Nano Res., 4, 7, 658 (2011). [ Links ]
[12] Z. Tang, C.-H. Tang and H. Gong, Adv. Funct. Mater., 22, 6, 1272 (2012). [ Links ]
[13] J.-H. Kim, R.-H. Kim and H.-S. Kwon, Electrochem. Commun., 10, 8, 1148 (2008). [ Links ]
[14] V. D. Jovic, B. M. Jovic and M. G. Pavlovic, Electrochim. Acta, 51, 25, 5468 (2006). [ Links ]
[15] L. D. Rafailovic, D. M. Minic, H. P. Karnthaler, J. Wosik, T. Trisovic and G. E. Nauer, J. Electrochem. Soc., 157, 5, D295 (2010). [ Links ]
[16] D. R. Gabe, J. Appl. Electrochem., 27, 8, 908 (1997). [ Links ]
[17] A. Brenner (Electrodeposition of alloys - principles and practice), Academic Press, New York and London (1963). [ Links ]
[18] R. Orinakova, A. Turonova, D. Kladekova, M. Galova and R. M. Smith, J. Appl. Electrochem., 36, 9, 957 (2006). [ Links ]
[19] Y. Gao, S. Chen, D. Cao, G. Wang and J. Yin, J. Power Sources, 195, 6, 1757 (2010). [ Links ]
Acknowledgments
The authors would like to acknowledge FCT for financial support under the project PTDC/CTM-MET/119411/2010- Electrodeposition of oxide spinel films on stainless steel substrates for the development of new electrodes for supercapacitors; the European Institute of Innovation and Technology, under the KIC InnoEnergy NewMat project and the COST Action MP 1004 Hybrid Energy Storage Devices and Systems for Mobile and Stationary Applications.
Artigo submetido em Fevereiro de 2013 e aceite em Abril de 2013
#First premium Portuguese Society of Materials, 2012.













