Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Corrosão e Protecção de Materiais
versão On-line ISSN 2182-6587
Corros. Prot. Mater. vol.31 no.3-4 Lisboa 2012
Corrosion behavior of Al and Al-Li alloys used as aircraft materials
Comportamento corrosivo de ligas de al e al-li usadas como materiais aeronaúticos
J. A. Moreto1,2, O. C. Gamboni 1, C. E. B. Marino3, W.W. Bose Filho1, J. C. S. Fernandes5,6 and L. A. Rocha 2,4
1 Department of Materials, University of São Paulo, Trabalhador Sãocarlense Av, 400, CEP: 13566-590, São Carlos/SP, Brazil.
2 CT2M – Centre for Mechanical and Materials Technologies, University of Minho, Campus de Azurém, P-4800-058, Guimarães, Portugal.
3 Department of Mechanical Engineering, Federal University of Paraná, CEP: 81531-990, Curitiba/PR, Brazil.
4 Department of Mechanical Engeneering, University of Minho, Campus de Azurém, P-4800-058, Guimarães, Portugal.
5 Department of Chemical Engineering, Instituto Superior Técnico, TULisbon, 1049-001 Lisboa, Portugal.
6 ICEMS, Instituto Superior Técnico, 1049-001 Lisboa, Portugal.
Corresponding author, e-mail: jeferson_moreto@yahoo.com.br
ABSTRACT
The aircraft industry is constantly looking for improved materials that offer benefits in terms of performance, weight and cost savings. The advantages of these alloys are lightweight, corrosion resistance, and very good thermal and electrical conductivity. In this paper, the corrosion resistance of two aluminum alloys was investigated by open circuit potential, potentiodynamic polarization and salt spray tests. The results suggest that the AA2198-T851 alloy tend to present a higher corrosion resistance when compared with the AA2524-T3 alloy. Consequently, in cases where corrosion is the main parameter to be considered, the AA2198-T851 may be a substitute for the AA2524-T3 alloy.
Keywords: Corrosion, Aluminum Alloys, Open Circuit Potential, Potentiodynamic Polarization, Salt Spray Tests
RESUMO
A indústria aeronáutica está constantemente à procura de materiais que oferecem benefícios em termos de desempenho, peso e custo. As vantagens destas ligas são a leveza, resistência à corrosão, boa condutividade térmica e eléctrica. Neste trabalho, foi investigada a resistência à corrosão de duas ligas de alumínio através de potencial de circuito aberto, polarização potenciodinâmica e ensaios de nevoeiro salino. Os resultados sugerem que a liga AA2198-T851 apresenta melhor resistência à corrosão quando comparada com a liga AA2524-T3. Consequentemente, em casos onde a corrosão é o principal parâmetro a ser considerado, a liga AA2198-T851 pode ser substituída pela liga AA2524-T3.
Palavras-chave: Corrosão, Ligas de Alumínio, Potencial de Circuito Aberto, Polarização Potenciodinâmica, Ensaios de Nevoeiro Salino
1. INTRODUCTION
Aluminum is used in virtually all segments of the aircraft, missile, spacecraft industry airframes, automotive engines and accessories. This is due to its high strength to density ratio, corrosion resistance, and weight efficiency, especially in compressive designs [1].
Aerospace industry has shown a renewed interest in Al-Li alloys. Since the discovery in the mid-1950s that lithium additions to aluminum alloys result in a material of high specific modulus, aluminum producers have diligently attempted to fabricate commercial alloys. The low ductility and fracture toughness of early Al-Li alloys have led to the significant amount of research invested in these alloys [1-3].
Corrosion of aluminum alloys has been the object of the study in numerous technical and scientific works, due to importance of these materials in the contemporary technical world [4].
The corrosion resistance of aluminum alloys depends on their composition metal heterogeneities and on the medium or exposure conditions. Corrosion in aluminum alloys essentially results from microgalvanic processes between different intermetallic phases and the matrix alloy [5].
The aluminum alloys may contain numerous different phases, which play an important role in corrosion pit formation [6]. In fact pitting is the most common form of corrosive attack in aluminum alloys. The progression of corrosion is caused by the potential difference between the anodic area inside the pit – which often contains acidic, hydrolyzed salts – and the surrounding cathodic area. Pits almost always initiate at some chemical or physical heterogeneity at the surface, such as inclusions, second-phase particles, flaws, mechanical damages, or dislocations.
In the 2xxx series aluminum alloys, the two major types of precipitates are denominated θ(Al2Cu) and S(Al2CuMg). The q is cathodic to the alloy matrix and causes corrosion of the aluminum alloy at the precipitate/alloy interface. The S phase is particularly susceptible to pitting corrosion [7]. These alloys are particularly sensitive to aqueous media containing chloride ion Cl-, which favor pitting corrosion, reducing the fatigue life, due to preferential stress concentration at pits [8-10].
The aim of this study was to evaluate the corrosion process of AA2198-T851 aluminum alloy, which is a promising substitute of the base line AA2524-T3 aluminum alloy normally used for aircraft fabrication. The effect of a saline environment (0.6 M NaCl) was considered.
2. EXPERIMENTAL
2.1 Materials
For the present investigations two aluminium alloys, named as AA2198-T851 and AA2524-T3 were used in the as received conditions. The weight compositions of the major elements in the studied alloys are presented in Table 1.
Table 1
Chemical composition of the aluminium alloys (wt%).
The AA2198-T851 aluminum alloy was developed for fuselage frames, while the AA2524-T3 is a relatively new Al-Cu-Mg alloy intended for fuselage skin replacements, presenting a superior fracture toughness and resistance to Fatigue Crack Growth (FCG).
2.2 Characterization
Samples from rolled sheets were cut and grounded using abrasive papers from 320# to 4000#. Prior to testing they were degreased with propanol and carefully cleaned with distilled water.
For the immersion corrosion tests, an aerated aqueous solution of 0.6 M NaCl was employed. After the tests, the surface morphology was studied by Scanning Electron Microscopy (SEM) using a Quanta 3D model FEG microscope. The microanalysis was performed by Energy Dispersive Spectroscopy (XEDS) using an EDAX Spectrometer, Phoenix Model, connected to the SEM microscope. For determination of Li element in the AA2198-T851 aluminum alloy an emission atomic spectroscopy Varian, Model MPX Vista was employed.
The aluminum alloys microstructures were characterized by XRD (CuKa radiation, continuous mode, scan step size of 0.04º, 2% weight detection limit, Bruker D8 Discover equipment).
2.3 Corrosion tests
Three types of corrosion tests were carried out: Open circuit potential monitoring (OCP), potentiodynamic polarization and salt spray tests. The corrosion potential (Ecorr) of the samples was monitored during 3 h. Potentiodynamic polarization followed the ASTM G61-09 standard and the measurements were made in the range -1.4 to 0.0 V with a potential sweep rate of 0.5 mV.s-1 using a GAMRY reference 600 potentiostat/galvanostat. All electrochemical experiments were conducted using a classical three-electrode configuration: the AA2198-T851 and AA2524-T3 aluminium alloys as working electrode with an exposed area of 0.38 cm2, a saturated calomel reference electrode, Hg/Hg2Cl2, KClsat and a platinum auxiliary electrode. The electrochemical experiments were made at (25 ± 1) oC.
Salt spray tests were carried out in accordance to ASTM B117-11 Standard in order to simulate a marine atmosphere. The prepared solution had a pH value of 6.5 – 7.2 and the inner chamber temperature was set to (35 ± 1) oC. Specimens were placed individually, parallel to each other, on plastic supports at an angle of orientation of 20o with regard to the vertical axis in order to provide uniform exposure of the surface to pitting corrosion.
After the exposure of the samples in the salt spray chamber, the specimen surface was covered with white corrosion products consisting of Al(OH)3. In order to analyze the surface, the corrosion products were rinsed with distilled water.
3. RESULTS AND DISCUSSION
3.1 Microstructure
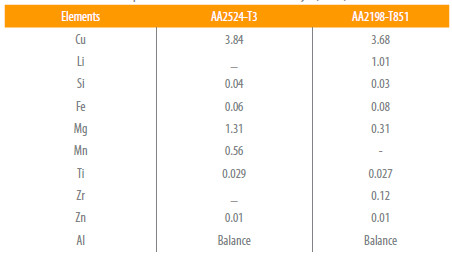
Fig. 1 presents the general microstructural features of the two aluminum alloys investigated in this work. Depending on the alloy chemical composition it is possible to find different types of precipitates at the grain boundaries and dispersed in the matrix. It can be observed that the alloys present an extensive fibering with elongated grains in the L direction. In the AA2524-T3 alloy the presence of non-metallic inclusions uniformly distributed in the material (small black points) was observed.
Figure 1
Microstructures general features from (a) AA 2198-T851 and (b) AA2524-T3 (200X).
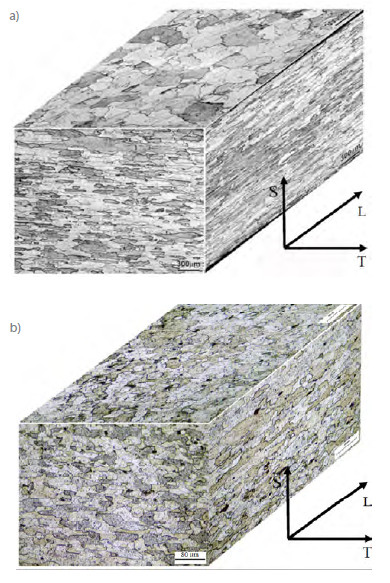
The following constituent phases of AA2198-T851 and AA2524-T3 aluminum alloys were identified in the XRD spectra (Fig. 2).
Figure 2
XRD pattern obtained in the (a) AA2198-T851 and (b) AA2524-T3 aluminum alloys.
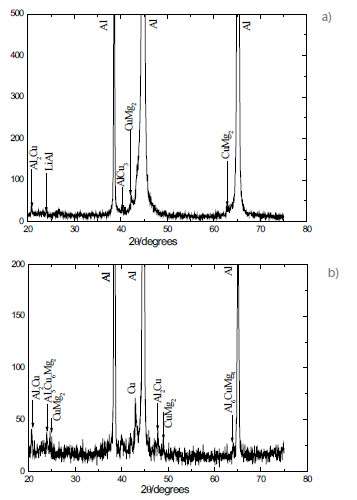
SEM observations of the two alloys further revealed the presence of intermetallic particles (Fig. 3). XEDS analysis provided their chemical composition. Probably the microstructure of AA2198-T851 aluminum alloy is composed by a dispersion of coherent precipitates T1 (Al, Mg, Fe, Cu). T1 is the main strengthening precipitate in some 2xxx series alloys, which precipitates preferentially at dislocations, subgrain and grain boundaries [11, 12]. These precipitates usually cause localized corrosion such as pitting corrosion and intergranular corrosion, due to the electrochemical potential difference between the precipitates and the matrix [13].
Figure 3
(a) T1 precipitate in AA2198-T851 aluminum alloy, (b) T1 precipitate in alloy AA2524-T3 aluminum alloy and (c) T2 precipitate in AA2524-T3 aluminum alloy.
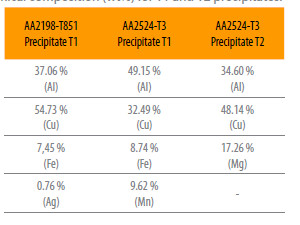
For the AA2524-T3 aluminum alloy, two types of coarse intermetallic particles were found: T1 (Al, Cu, Mn and Fe) and incoherent precipitates T2 (Al, Cu, Mg). The wt% compositions of the T1 and T2 precipitates in the studied alloys are presented in Table 2.
Table 2
Chemical composition (wt%) for T1 and T2 precipitates.
3.2 Open circuit potential and potentiodynamic polarization
Representative curves of the OCP evolution with the immersion time for the AA2198-T851 and AA2524-T3 aluminium alloys are presented in Fig. 4. The results show that the electrochemical potential of the AA2524-T3 is more positive than that observed in the AA2198-T851 aluminium alloy, that could indicate a lower susceptibility to corrosion. However, the main reason for the lower potential values recorded for AA2198-T851 should be the presence of Li, a highly reactive metallic element that may be responsable for the decrease of the potential.
Figure 4
Open circuit potential curves for AA2198-T851 and AA2524-T3 aluminum alloys, t = 3 h.
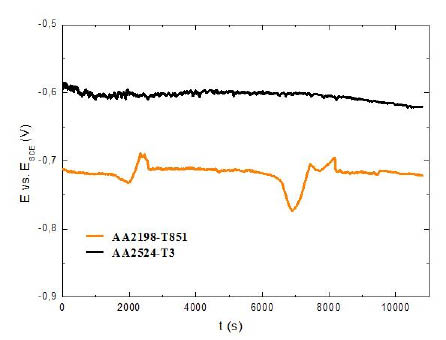
In this context, it is possible to assume that the dependence of potential with time is linked to the stochastic evolution of pitting events and to the resulting variations in the ratio of areas covered/uncovered and/or cathode/anode, which may be related to the presence of different types of intermetallics.
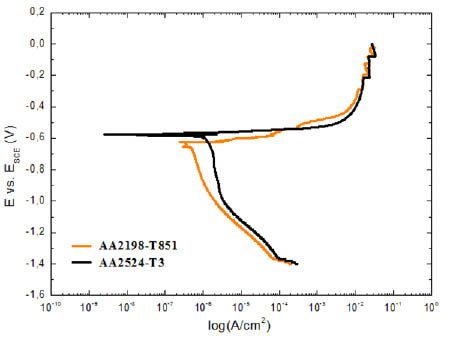
The characteristic potentiodynamic polarization curves obtained for the AA2198-T851 and AA2524-T3 aluminium alloys are presented in Fig. 5. As it can be seen, the AA2524-T3 alloy presented a corrosion potential (Ei=0) slightly more positive than that measured in the AA2198-T851 aluminium alloy. No passive plateau can be observed in the anodic domain of both alloys, suggesting a growth of the pit with the surge current. Also, as it can be deducted from the polarization curves, the corrosion current density (icorr) is limited by the cathodic behaviour, which is higher for AA2198-T851 aluminium alloy.
Figure 5
Polarization plots obtained for AA2198-T851 and AA2524-T3 aluminum alloys in 0.6 M NaCl solution, v = 0.5 mV.s-1.
According to Birbilis et al. [14] the corrosion resistance of aluminum alloys is influenced by the presence of intermetallic compound particles exhibiting different electrochemical characteristics from those of the matrix. When the intermetallic compound particles are nobler than the matrix, circumferential pits appear as a circle of attack around the more or less intact particle.
The presence of precipitates as T1 and T2 results in an inversion of the corrosion mechanism. First of all, these precipitates are anodic, resulting in the dissolution of them. However, during the corrosion process Li is preferentially dissolved resulting in an increase of the amount of Cu in the surface. Consequently, the corrosion potential rises to more positive values, causing the dissolution of the matrix.
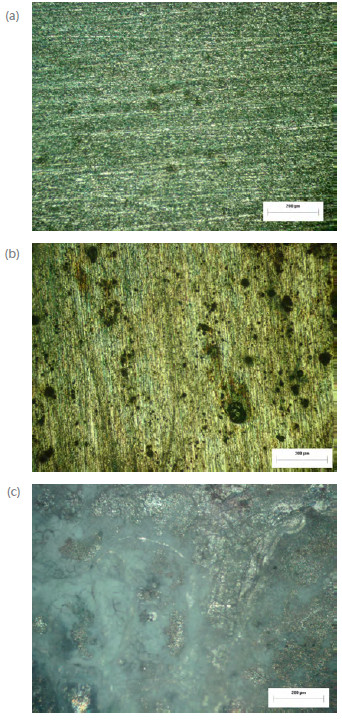
Fig.s 6 and 7 show the surface morphology of AA2198-T851 and AA2524-T3 aluminium alloys after 48 h exposure in a salt spray chamber. A high surface density of small pits can be detected on both alloys already after 3 h of exposure. By the 24th hour of immersion the pit size has increased significantly and after 48 h the surface has become covered by a thick layer of corrosion products. It is well known that pitting corrosion has a strong effect on the fatigue life of aluminium alloys used in aircraft structures. Corrosion can lead to accelerated failure of structural components under fatigue loading conditions. Understanding and prediction corrosion damage is very important for the structural integrity of aluminium alloys and structures.
Figure 6
AA2198-T851 aluminium alloy exposed to 5 % NaCl solution in salt spray tests, (a) 3 h, (b) 24 h and (c) 48h (100X).
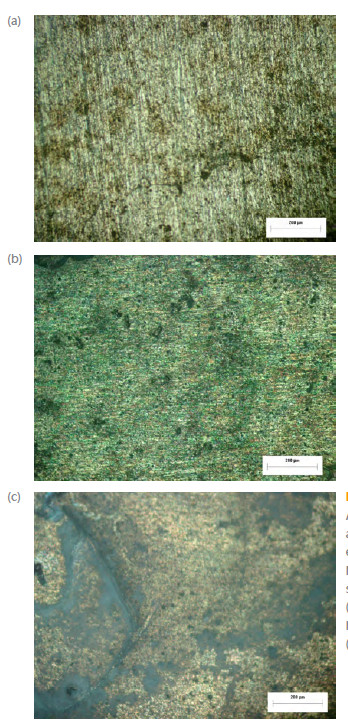
Figure 7
AA2524-T3 aluminium alloy exposed to 5% NaCl solution in salt spray tests, (a) 3 h, (b) 24h and (c) 48 h (100X).
4. CONCLUSIONS
The studied aluminum alloys presented different precipitates types depending on alloy chemistry, as well as different grain morphology depending on the thermomechanical processing employed. The potentiodynamic polarization curves suggested that the AA2198-T851 alloy tend to present a higher corrosion resistance when compared with the AA2524-T3 alloy. Consequently, in cases where corrosion is the main parameter to be considered, the AA2198-T851 may be a substitute for the AA2524-T3 alloy.
REFERENCES
[1] ASM Metals Handbook (Properties and Selection: Nonferrous Alloys and Special - Purpose Materials), v. 2, ASM International, Ohio, p. 17 (1990). [ Links ]
[2] Jr. T. H. Sanders and Jr. E. A. Starke (Aluminum-Lithium Alloy), Metallurgical Society of AIME, USA (1981). [ Links ]
[3] Jr. T. H. Sanders and Jr. E. A. Starke (Aluminum-Lithium Alloys 11) in Proceedings of 2nd International Conference on Aluminum-Lithium Alloys, April, Monterey, CA (1983). [ Links ]
[4] A. Banu, M. Marcu, O. Radovici, C. Pirvu and M. Vasilescu, Revue Roumaine de Chimie, 51(3), 193 (2006). [ Links ]
[5] K. Nisancioglu (Corrosion of aluminium alloys) in Proceedings of 3rd International Conference on Aluminium Alloys, V. 3, p. 239, Trondheim, Norway (1992). [ Links ]
[6] Q. Y. Wang, R. M. Pidapart and M. J. Palakal, AIAA Journal, 39, 2 (2001). [ Links ]
[7] R. Grilli, M. A. Baker, J. E. Castle, B. Dunn and J. F. Watts, Corros. Sci., 52, 2855 (2010). [ Links ]
[8] J. R. Davis (Metals Handbook), v. 13, ASM International, Ohio, USA, p.104 (1987). [ Links ]
[9] E. H. Hollingsworth, H. Y. Hunsciker and P. A. Schweitzer (Aluminium alloys) in Corrosion and Corrosion Protection Handbook, (Philip A. Schwitzer, ed.) 2nd Ed., p.135 (1983). [ Links ]
[10] E. H. Hollingsworth and H. Y. Hunsciker (Corrosion of aluminum and aluminum alloys) in Corrosion, v. 13, ASM Handbook, American Society of Materials International, Materials Park, Ohio, USA, p.583 (1987). [ Links ]
[11] K. S. Kumar, S. A. Brown and J. R. Pickens, Acta Mater., 44(5), 1899 (1996). [ Links ]
[12] P. Niskanen, T. H. Sanders and J. G. Kinker, Corros Sci., 22(4), 283 (1982). [ Links ]
[13] J. F. Li, C. X. Li, Z. W. Peng, W. J. Chen and Z. Q. Zheng, Journal of Compounds, 460, 688 (2008). [ Links ]
[14] N. Birbílis and R. G. Buchheit, J. Electrochem. Soc., 152(4), 140 (2005). [ Links ]
Artigo submetido em Setembro de 2011 e aceite em Agosto de 2012
ACKNOWLEDGEMENTS
The authors would like to thank CAPES Process: BEX4936/10-8, Centre for Mechanical and Materials Technologies of University of Minho and Department of Materials - University of São Paulo for financial support.













