Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Corrosão e Protecção de Materiais
versão On-line ISSN 2182-6587
Corros. Prot. Mater. vol.31 no.2 Lisboa 2012
Synthesis and characterization of new hybrid copolymers for high performance coatings application
Sintese e caracterização de novos copolímeros híbridos para aplicação de revestimentos de elevado desempenho
A. Carvalho1, E. Veludo2, J. Machado2, J. F. J. Coelho1 and M. H. Gil1
1 Chemical Engineering Department, Faculty of Science and Technology of University of Coimbra, Pólo 2, 3030-290 Coimbra, Portugal.
2 CIN – Corporação Industrial do Norte, S.A., Avenida Dom Mendo nº 831, Apartado 1008, 4471-909 Maia, Portugal.
Corresponding author, e-mail: hgil@eq.uc.pt
ABSTRACT
Polymeric hybrid materials and their specific functionalization have attracted great interest, due to their chemical, thermal and mechanical properties performance, in relation to their equivalent non hybrids. Siloxane based materials offer great interest due to their excellent chemical, physical and electric properties. The primary structure of the siloxane could be modified either by side chain functionalization or by changing the main chain, forming hybrid materials. This kind of materials could be used in several and diverse areas, namely in protective and high performance coatings. The enhancement of their properties can be achieved by incorporating materials such as polyhedral oligomeric silsesquioxanes (POSS) into their polymeric chain. In this paper are presented two new hybrid polymers, the poly(methyl methacrylate–co-isobutyl acrylate–co-glycidyl methacrylate–co-vinyltriethoxysilane) (POSS_0) and the poly(methyl methacrylate–co-isobutyl acrylate-co-glycidyl methacrylate-co-vinyltriethoxysilane–co–(isobutyl)propyl methacrylate(polisilsesquioxane)(POSS_15). These copolymers were prepared by a radical polymerization, and were designed to have the same structure acrylic, epoxy, silane and POSS fractions, which lead to offer multiple properties with high interest to coating systems due to their epoxy and siloxane groups.
The presence of the POSS cage in the hybrid polymeric structure increases the hydrophobic character and the thermal stability. When applied in commercial bi-component anti-corrosive paint formulations, the hybrid copolymers presented good performance, good compatibility properties for the different formulation components without forming agglomerates or phase separation. The coating formulation shows quick drying. These materials presented excellent mechanical properties, good adhesion, flexibility and impact resistance.
Keywords: Hybrid Materials, Radical Polymerization, POSS, High Performance Coatings
RESUMO
Devido à superioridade das suas propriedades químicas, térmicas e mecânicas, os materiais poliméricos híbridos têm atraído bastante interesse comparativamente aos materiais equivalentes não híbridos. Os materiais de base siloxano concentram grande interesse devido às suas excelentes propriedades químicas, físicas e elétricas. A sua estrutura primária pode ser alterada através de funcionalização das cadeias laterais, ou alteração da cadeia principal, obtendo deste modo materiais híbridos. A aplicação destes híbridos pode ser bastante diversificada: em áreas como revestimentos de elevado desempenho ou para proteção. O incremento das suas propriedades pode ser feito através da incorporação de materiais do tipo polisilsesquioxano (POSS) na cadeia principal. Neste trabalho pretende-se apresentar dois novos copolímeros híbridos: o poli(metacrilato de metilo-co-acrilato de isobutilo-co-metacrilato de glicidilo-co-viniltrietoxisilano) (POSS_0) e o poli(metacrilato de metilo-co-acrilato de isobutilo-co-metacrilato de glicidilo-co-viniltrietoxisilano-co-metacrilato de (isobutil)propilpolisilsesquioxano (POSS_15). Estes copolímeros foram preparados através de uma polimerização radicalar, obtendo-se na mesma estrutura as funcionalidades acrílico, epóxido, silano e POSS. A presença das funcionalidades epóxido e silano na mesma estrutura tem elevado interesse para aplicação em revestimentos.
A presença de grupos do tipo POSS, na estrutura polimérica híbrida, aumenta o carácter hidrofóbico do material e a sua estabilidade térmica. Ao aplicá-los num sistema comercial bi-componente para proteção anticorrosiva, verificou-se um bom desempenho, nomeadamente boa compatibilidade entre os componentes da formulação, e boa dispersão, sem a ocorrência de aglomerados. Os revestimentos obtidos apresentaram uma secagem rápida, bem como excelentes propriedades mecânicas, boa adesão, flexibilidade e resistência ao impacto.
Palavras chave: Materiais Híbridos, Polimerização Radicalar, POSS, Revestimentos de Elevado Desempenho
1. INTRODUCTION
Polymeric hybrid materials have attracted great interest due to their chemical, thermal and mechanical properties, when compared to their equivalent non hybrids polymers. These materials combine the advantageous properties of the organic materials, such as toughness, elasticity, durability and processability, with the interesting properties of the inorganic materials, such as hardness, chemical resistance and weatherability [1-4].
Silane based hybrids or composite materials offer suitable properties for protective coatings applications due to their excellent chemical, physical and electric properties. However, the weak interactions between silane and organic molecules avoid the formation of fibers and films. In order to overcome this problem, the primary structure of the siloxane can be modified either by side chain functionalization or by changing the main chain, forming covalent hybrid materials [5].
The preparation of these hybrid materials requires controlling the homogeneity of the two components [1]. This mixture between organic and inorganic phases could be obtained by incorporating acrylic polymers or oligomers into the inorganic matrix, by a simple physical mixture of the different parts (forming composites [6]) or by chemical polymerization of the acrylic and silane monomers (forming hybrid co-polymers [3, 5]).
Metallic corrosion phenomena occur via chemical reaction between the metal surface and the different species in the environment. Species as chloride or sulfate ions, oxygen and water, combined with the electron transport in the metal play an important action in the corrosion process [7-10]. In order to prevent the corrosion damage, different coating systems can be used. Depending on the most convenient option to each case, barrier coatings, inhibitive coatings and zinc-rich coatings can be applied [9, 11, 12].
Among several polymers used for anti-corrosive coatings, epoxy are suitable polymers due to their good mechanical and adhesion properties, excellent surface adhesion to metals (and other substrates) and good water, chemical and acid resistances [5, 13]. The epoxy, oxirane ring provides a site for crosslinking with proton donor species, such as amines, carboxyl and hydroxyl groups [13].
However epoxy resins present also some limitations since they are very sensitive to UV degradation, tend to lose gloss, present low thermal stability and low pigment binding stability and are hydrophobic. The copolymerization of epoxy resins with acrylic polymers, that brings other properties such as UV resistance, is an important route to mitigate the described problems [5, 13].
From a commercial standpoint epoxy materials are attractive since they are more effective against corrosion in thinner films than most other finishing materials. They are often used as primers in association with other materials which have good barrier properties but marginal adhesive characteristics. Epoxy polymers can be used in different types of formulations: one-component products that require high curing temperatures or using two-component being possible to promote the curing at ambient temperature [14].
In polymeric hybrid coatings, the epoxy properties could be combined with those of acrylic and silanes. Therefore, it is possible to combine in the same material the typical crosslinking density of epoxies, the heat resistance and superior weathering and water resistance of the silane, as the wide range of flexibility and the good UV stability from the acrylics [13, 15]. The use of fillers as polyhedral oligomeric silsesquioxanes (POSS) can also improve the temperature and oxidation resistance, the surface hardening, decrease the flammability and the mechanical resistance of the hybrid materials [16].
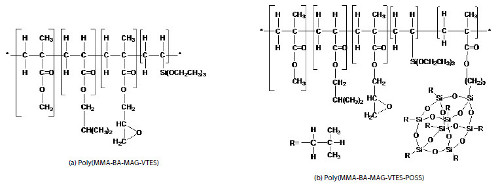
In this study an epoxy-acrylic-silane hybrid copolymer was synthesized (Fig. 1). Each block segment of the hybrid copolymer is expected to play a specific role in the properties of the copolymer: the hardness of the material will be given by the methyl methacrylate (MMA), the flexibility by the isobutyl acrylate (BA), the reactive sites by the glycidyl methacrylate (MAG), and the adhesion properties by the vinyltriethoxysilane (VTES). The purpose of the methacrylate isobutyl POSS (iBu-POSS) monomer addition is to increase thermal stability of the final polymeric material and due to its compatibility with the other co-monomers in the system.
Figure 1
Chemical structures proposed for the hybrid copolymers prepared.
2. EXPERIMENTAL
2.1. Materials
Methyl methacrylate (MMA), isobutyl acrylate (BA) and glycidyl methacrylate (MAG) were purchased from Sigma Aldrich.
To the storage of the monomers it is added an stabilizer/inhibitor, namely hydroquinone, which must be removed before use. The inhibitor was removed from the MMA and BA monomers by a liquid/liquid extraction with an aqueous solution of sodium hydroxide (NaOH) and sodium chloride (NaCl), and from the MAG by passing through a column containing as stationary phase sand/alumina/sand. Vinyltriethoxysilane (VTES) was purchased from Sigma Aldrich and methacrylate isobutyl POSS (iBu-POSS) was purchased from Hybrid Plastics Company and used as received.
Benzoyl peroxide (BPO) was purchased from ACROS Company and used as received. A mixture of p-xylene isomers and dodecanethiol were ordered from Sigma Aldrich and used as received.
The hardener, an amino functional methyl phenyl silicone resin solution, was purchased from Wacker and used as received.
2.2. Synthesis methodology
The hybrid copolymers were prepared by solution polymerization, using as radical initiator BPO. The synthesis procedure was adapted from the work of [17], as following: a p-xylene solution (60 mL) containing 700 mg of BPO and 1 mL of dodecanethiol (as chain transfer agent) were introduced in a 250 mL four-necked flask. The system was purged during 30 min with N2.
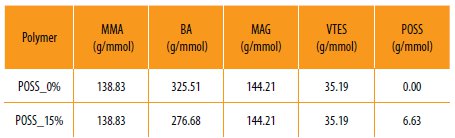
Then, 10 mL of the previously purified monomers mixture (Table 1) was added to the reaction vessel. The mixture was heated up to 75 ºC. Once the reaction temperature was reached 5 mL of the monomers mixture was added dropwise to the mixture during 2 h under N2 atmosphere. The reaction was kept over reflux for more 3 hours, resulting in a viscoelastic solution.
Table 1
Monomer quantities used to prepare the several hybrid polymers.
2.3. Characterization
The evolution of the polymeric reaction, as well as the chemical composition of the hybrid copolymers films were evaluated by Fourier Transformed Infrared Spectroscopy (FTIR). The FTIR spectrum was recorded using a Spectrum BX (Perkin Elmer) equipped with a Total Attenuated Reflectance Golden Gate horizontal cell (Specac), in the range 4000-500 cm-1 with a resolution of 4 cm-1. An average of 124 scans were recorded to increase the signal to noise ratio.
The chemical structure of the monomers and final hybrid copolymers were evaluated by 1H-NMR. 1H-NMR spectra were obtained at 25 ºC on a Varian Unity 600 MHz Spectrometer using a 3 mm broadband NMR probe, in deuterated tetrahydrofuran (THF-d8).
Contact angle measurements were carried out by using a Data Physics OCA 20 apparatus.
In order to evaluate the surface free energy of the several hybrid copolymers prepared, static contact angles were measured with four standard liquids: distilled water, formamide, ethylene glycol and propylene glycol. The reported contact angle values were determined by average of 7 measurements at room temperature using the sessile drop method. Surface free energy values, as well as the dispersive and polar energy components, were obtained according the Owens-Wendt-Rabel and Kaelbe method (OWRK) [18].
The thermal stability of the hybrid copolymers prepared was evaluated by termogravimetric analysis (TGA). The TGA curves of the hybrid films were recorded using a SDT Q100 (TA Instruments) thermal analysis system. With this aim 3 mg samples of the hybrid copolymers were placed into platinum pans and heated from 25 ºC to 600 ºC, with a 10 ºC.minute-1 heating rate.
The particle size of the grind obtained was evaluated by a Hegmann Gauge, to get the desired fineness value around 25 µm (NP EN ISO 1524) [19].
The viscosity of the copolymer paint at (23±1) ºC was evaluated with a Brookfield Viscometer, using a R5 spindle at 20 rpm, according to ASTM D2196 [20].
2.4. Formulation of hybrid co-polymers based paints
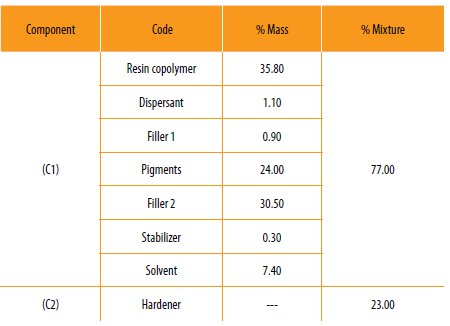
The hybrid copolymers prepared in this work were used to prepare a two component anticorrosive paint. The first component is the paint containing the hybrid copolymer in a standard formulation (Table 2).
The hybrid copolymer, dispersants, fillers, solvent and pigments were placed in a ball mill machine and homogenized with a high speed Cowles disperser for 20 minutes. The first component was mixed with the hardener in the proportion of 3:1. The final viscosity of the mixture was evaluated.
Table 2
Composition of the hybrid co-polymer epoxy resin, first component of the anticorrosive bi-component paint and the hardener.
3. RESULTS AND DISCUSSION
3.1. Hybrid copolymers chemical characterization by atr-ftir
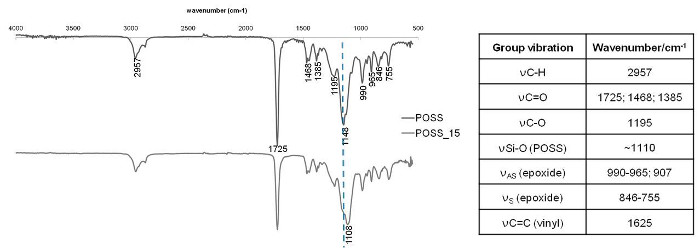
The extension of the polymerization reaction was evaluated by FTIR. The absorbance of the functional groups from the monomers and from the hybrid copolymers was measured in the range 4000-500 cm-1. The FTIR spectra obtained for the two hybrids materials, POSS_0 and POSS_15, are presented in Fig. 2. From the analysis of the Fig. 2 it is possible to verify the absence of the band corresponding to the double bond, at ~1625 cm-1, which suggest no residual monomer in the mixture. It is also possible to observe the displacement to lower wavenumbers, of the band maximum from 1148 cm-1 to 1108 cm-1 corresponding to the POSS_0 and POSS_15 respectively. This displacement could be dependent on the influence from the vibrations due to the POSS cage in the copolymer structure. It is also possible to observe the presence of the glycidyl oxirane ring symmetrical and asymmetrical vibration bands in the shoulder present at 1250 cm-1 and in the bands present at 950-810 cm-1 respectively [21-24].
Figure 2
Infrared spectra obtained for the hybrid copolymers prepared (POSS_0 and POSS_15) and theoretical vibration bands for this kind of chemical groups.
3.2. Chemical characterization by 1H-NMR
From the spectrum obtained for the POSS_0 (Fig. 3) it is possible to observe the presence of the epoxy group protons (d=3.19 ppm, d=2.26-2.46 ppm) and the silane protons at (d=0.95 ppm, d=1.07 ppm and d=3.90 ppm). Following the results obtained by FTIR no peaks related vinyl monomer groups at d=4.5-5 ppm has been detected.
Figure 3
1H-NMR spectrum of the POSS_0 and POSS_15 hybrid copolymers.
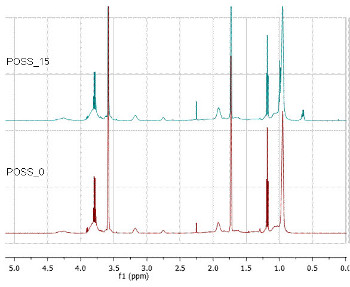
From the spectrum obtained for the POSS_15 (Fig. 3) it is possible to ascribe at d=3.19 ppm and d=2.75 ppm the signals from the protons of the epoxy group and the signals from the ethoxy group, linked to the silane, at (d=3.78 ppm; d=1.10 ppm and d=0.95 ppm).
Since the hybrid copolymers prepared only differ in the presence or absence of the iBu-POSS co-monomer, it is possible to compare the obtained spectra. By overlapping the POSS_0 and POSS_15 spectra (Fig. 3), some differences were found, namely, the increase of the signal at d=2.26 ppm (from the –(CH2)2- protons of the iBu-POSS monomer) and the new signal at d=0.65 ppm corresponding to the –CH2– protons linked directly to the POSS cage [25-27].
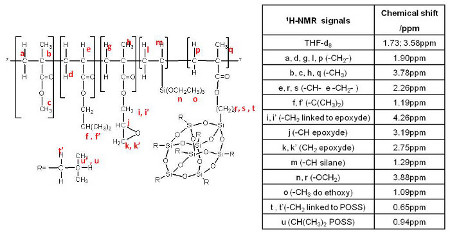
The Fig. 4 shows the assignment of the different NMR peaks for the POSS_15 copolymer.
Figure 4
1H-NMR signals identification for the POSS_15 hybrid copolymer.
3.3. Surface energy determination by contact angle measurement
The films used for the determination of the surface energy were prepared by solvent casting (by using acetone).
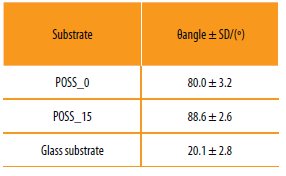
A glass substrate was used as standard, since its surface is considered hydrophilic (θangle ~20-30º) [28].
The static contact angles obtained are presented in Table 3. The results suggest that the copolymers prepared are hydrophilic being the variation of contact angle due to incorporation of POSS residual.
Table 3
Results obtained for the static contact angles with water (θWater).
In order to understand the influence of the incorporation of the POSS in the hybrid systems, the surface energy was determined. The surface free energy was calculated based on the static contact angles obtained for several probe liquids by using the OWRK (Owens-Wendt-Rabel and Kaelble) model, which consider both the dispersive and polymer contributions [18].
The results obtained for the surface energy are presented in Table 4.
Table 4
Results obtained for the total surface tension (γ), dispersive component (γd) and polar component (γp) of the hybrid copolymers prepared.
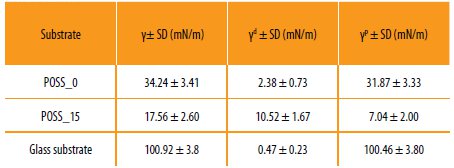
It is possible to observe that the incorporation of the POSS into the hybrid copolymer system decreases the surface energy, which is related with the increase of the dispersive component. This effect is very important in coatings for protective applications [29].
3.4. Thermal behaviour
The thermal stability of the hybrid copolymers prepared was evaluated by termogravimetric analysis (TGA). The results obtained to the POSS_0 and POSS_15 hybrid copolymers, are presented in the Fig. 5 and in Table 5.
Figure 5
Overlap of the TGA curves for the hybrid copolymers (POSS_0 and POSS_15) and for the iBu-POSS monomer.
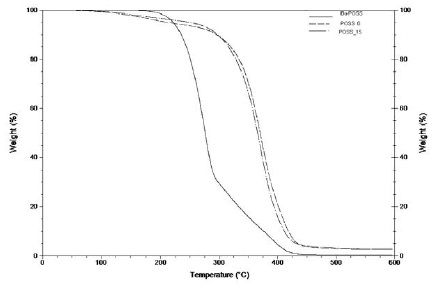
Table 5
Results obtained for the termogravimetric analysis (TGA) of the hybrid copolymers.
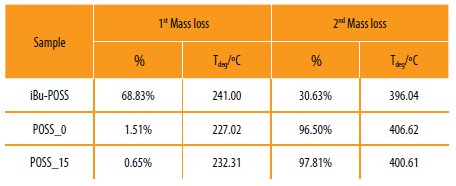
The POSS_0 and POSS_15 prepared, show high thermal resistance (start to degrade only around 225 ºC), when compared with the conventional epoxy systems [30]. The POSS incorporation in the hybrid polymeric systems decreases the degradation temperature (406 ºC to ~400 ºC) but increases the thermal stability of the material (1st degradation: 1,5 % to 0,65%). This behavior could be related to the presence of the POSS cage into the system. The POSS cage is formed by Si-O-Si covalent bonds, which presents higher bond dissociation compared to the Si-O and C-C bonds [5, 31].
This fact confirms that the POSS monomer was successfully covalent bonded to the polymeric structure. The presence of the POSS cage in the polymeric structure acts as an internal filler which promotes the stability of the final material [31].
3.5. Coating performance evaluation
The hybrid copolymer formulations (POSS_0 and POSS_15) were mixed with the hardener. The two mixture were homogenized for 5-10 minutes.
The final bi-component paint was used to coat: a) glass- to test brightness (NP EN ISO 2813) [32] and drying time (ASTM D 1640) [33], b) metal- to test adhesion (NP EN ISO 2409) [34], impact resistance (NP EN ISO 6272) [35] and flexibility (ASTM D 522) [36] and c) Leneta charts- to test the yellowness and whiteness index (ASTM E 313) [37]. All the coated materials present standard sizes.
The main results obtained are presented in Table 6.
Table 6
Summary of the characteristics of the hybrid copolymers based paints.
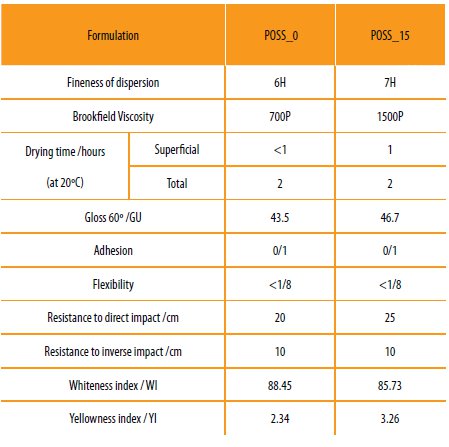
The paints formulated with the hybrid copolymers present good dispersion, no agglomeration or phase separation, which indicates that the copolymers were compatible with the other components of the system.
The viscosity of the paints increased due to the presence of the POSS cage structure in the copolymer. It is known that the presence of the POSS cage, a rigid structure that occupies a very large space into the polymeric structure, narrows the molecules mobility into the polymeric chain increasing the viscosity of the system.
After spreading the paint on a glass surface the drying time was evaluated (superficial and complete drying). Both films have a total drying time around 2 hours, showing the occurrence of cross-linking reaction with the hardener.
The paint gloss value obtained was very low, which could be related to the amorphous character of the hybrid copolymer.
The higher impact resistance, flexibility and adhesion properties when the new coatings were applied on the metal could be related to the dense structure promoted by the hybrid copolymer, which has oxirane and siloxane moieties in the polymeric chain. In principle these groups can react with the hardener forming a dense, highly adhered coating structure. The system with the POSS cage (POSS_15) presents a better resistance to direct impact. These properties are due to the presence of Si-O linkages, which are by nature flexible and mobile [5].
The incorporation of the POSS cage into the hybrid co-polymer decreases the whiteness index and increases the yellowness index of the final coating, comparatively to the results obtained for the POSS_0 system. This fact could be related to the interaction of the POSS cage with the pigments, once both are inorganic [38].
4. CONCLUSION
This work shows the possibility of preparing two new hybrid copolymers by using solution polymerization. The copolymers were designed to have the same structure acrylic, epoxy, silane and POSS fractions, which lead to offer multiple properties with high interest to coating systems.
The presence of the POSS cage in the hybrid polymeric structure increases the hydrophobic character and the thermal stability.
These new materials present high potential in high performance protective coatings application due to their epoxy and siloxane groups. The epoxy groups are widely used as crosslinking binder, promoting the formation of high density coatings. The silane groups, due to their sol-gel reaction character, improve the coating adhesion behavior to metal substrates. The combination of epoxy and silane functionalities in the same polymer, will increase the coating final performance.
When applied in commercial bi-component anticorrosive paint formulations, the hybrid copolymers presented good performance. Good compatibility properties for the different formulation components were observed, without forming agglomerates or phase separation. The coating formulation shows quick drying (2 hours), however such time is synonym of a short pot-life.
These materials showed excellent mechanical properties, good adhesion, flexibility and impact resistance.
In conclusion, the results presented in this work suggest that the new hybrid copolymers have high potential as protective coatings, namely as anticorrosive coatings, fact that must be proved in the next phase of the work, already initiated.
REFERENCES
[1] T. Wan, J. Lin, X. Li and W. Xiao, Polym. Bull., 59, 749 (2008). [ Links ]
[2] J. M. Keijman (Properties and use of inorganic polysiloxane hybrid coatings for the protective coatings industry) in Proceedings of 2nd Journeys of Journal Corrosão e Protecção de Materiais, November, Lisboa, Portugal, (2000). [ Links ]
[3] L. Wenbo, Q. Jinqing, L. Zhong and C. Huanqin, Prod. Eng. Chem. Tech., 18, 156 (2010). [ Links ]
[4] C. Sanchez, L. Rozes, F. Ribot, C. Laberty-Robert, D. Grosso, C. Sassoye, C. Boissiere and L. Nicole, C. R. Chemie, 13, 3 (2010). [ Links ]
[5] S. Ahamad, A. P. Gupta, E. Sharmin, M. Alam and S. K. Pandey, Prog. Org. Coat., 54, 248 (2005). [ Links ]
[6] M. E. P. Sousa, E. Ariza, M. Ballester, I. V. P. Yoshida, L. A. Rocha and C. M. A. Freire, Materials Research, 9, 59 (2006). [ Links ]
[7] L. M. Abrantes, A. S. Viana, Corros. Prot. Mater., 28, 1, p.6 (2009). [ Links ]
[8] Z. Lourenço, Corros. Prot. Mater., 26, 3, p.79 (2007). [ Links ]
[9] C. H. Hare (Corrosion and its control by coatings), in Coatings Materials and Surface Coatings. (A. A. Tracton, ed.), Taylor and Francis Group, London, p.1 (2007). [ Links ]
[10] V. H. V. Sarmento, M. G. Schiavetto, P. Hammer, A. V. Benedetti, C. S. Fugivara, P. H. Suegama, S. H. Pulcinelli and C. V. Santilli, Surf. Coat. Tech., 204, 2689 (2010). [ Links ]
[11] M. L. Zheludkevich, I. M. Salvado and M. G. S. Ferreira, J. Mater. Chem., 15, 5099, (2005). [ Links ]
[12] D. Wang and G. P. Bierwagen, Prog. Org. Coat., 64, 327 (2009). [ Links ]
[13] A. Forsgren (Composition of the anticorrosion coating), in Corrosion control through organic coatings, Taylor and Francis Group, London, p.11 (2006). [ Links ]
[14] E. M. Petrie, (Epoxy adhesives), in Epoxy adhesive formulations, Mc Graw-Hill, New York (2006). [ Links ]
[15] G. Reusmann, Macromol. Symp., 187, 235 (2002). [ Links ]
[16] D. Gnanasekaran, K. Madhavan and B. S. R. Reddy, J. Sci. Ind. Res., 68, 437 (2009). [ Links ]
[17] T. Y. Guo, C. Xi, G. J. Hao, M. D. Song, H. Z. Zhang, Adv. Polym. Tech., 24, 288 (2005). [ Links ]
[18] S. Pinto, P. Alves, C. P. Matos, A. C. Santos, L. R. Rodrigues, J. A. Teixeira e M. H. Gil, Colloid. Surface, B, 81, 20 (2010). [ Links ]
[19] NP EN ISO 1524: 2007 (Tintas e vernizes. Determinação da finura de moagem), IPQ, Caparica, Portugal (2007). [ Links ]
[20] ASTM D2196-10 (Standard Test Methods for Rheological Properties of Non-Newtonian Materials by Rotational (Brookfield type) Viscometer), ASTM International, PA, USA (2010) [ Links ]
[21] E. Tegou, V. Bellas, E. Gogolides and P. Argitis, Microelectron. Eng., 73, 238 (2004). [ Links ]
[22] E. Markovic, S. Clarke, J. Matisons and G. P. Simon, Macromolécules, 41, 1685 (2008). [ Links ]
[23] B. Stuart (Polymers) in Infrared spectroscopy: Fundamentals and applications, ACOL Series, Wiley, Chichester, UK, p.113 (2004). [ Links ]
[24] C. Ramírez, M. Rico, A. Torres, L. Barral, J. López and B. Montero, Eur. Polym. J., 44, 3035 (2008). [ Links ]
[25] S. Ahmad, A. P. Gupta, E. Sharmin, M. Alam and S. K. Pandey, Prog. Org. Coat., 54, 248 (2005). [ Links ]
[26] H.-S. Park, I.-M. Yang, J.-P. Wu, M.-S. Kim, H.-S. Hahm, S.-K. Kim, and H.-W. Rhee, J. Appl. Polym. Sci., 81, 1614 (2001). [ Links ]
[27] K. D. Safa and M. H. Nazirtabrizi, Polym. Bull., 57, 293 (2006). [ Links ]
[28] K.-B. Lim and D.-C Lee., Surf. Interface Anal., 36, 254 (2004). [ Links ]
[29] G. X. Shen, Y. C. Chen, L. Lin, C. J. Lin and D. Scantlebury, Electrochim., Acta, 50, 5083 (2005). [ Links ]
[30] K. Pielichowski and J. Njuguna, (Organic-inorganic hybrid polymers) in Thermal degradation of polymeric materials, Rapra Technology Limited, Crewe, UK, p.184 (2005). [ Links ]
[31] A. Fina, D. Tabuani, F. Carniato, A. Frache, E. Boccaleri and G. Camino, Thermochim. Acta, 440, 36 (2006). [ Links ]
[32] NP EN ISO 2813 : 2001 (Tintas e vernizes - Determinação do brilho especular de revestimentos por pintura não metálicos a 20º, 60º e 85º), IPQ, Caparica, Portugal (2001). [ Links ]
[33] ASTM D1640 - 03: (2009) (Standard Test Methods for Drying, Curing, or Film Formation of Organic Coatings at Room Temperature), ASTM International, PA, USA, (2009). [ Links ]
[34] NP EN ISO 2409: 1995 (Tintas e Vernizes - Aderência pelo método da quadrícula), IPQ, Caparica, Portugal (1995). [ Links ]
[35] NP EN ISO 6272-2: 2008 (Tintas e vernizes - Ensaios de deformação rápida (resistência ao choque) Parte 2: Ensaio de queda de uma massa com pequena área de indentação), IPQ, Caparica, Portugal (2008). [ Links ]
[36] ASTM D522-93a (2001) (Standard Test Methods for Mandrel Bend Test of Attached Organic Coatings), ASTM International, PA, USA (2001). [ Links ]
[37] ASTM E313 - 98 (Standard Practice for Calculating Yellowness and Whiteness Indices from Instrumentally Measured Color Coordinates), ASTM, Internationel, PA, USA (1998). [ Links ]
[38] J. Zhou and J. Kieffer. J. Phys. Chem. C, 112, 3473 (2008). [ Links ]
Artigo submetido em Setembro de 2011 e aceite em Maio de 2012













