Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Corrosão e Protecção de Materiais
versão On-line ISSN 2182-6587
Corros. Prot. Mater. vol.31 no.1 Lisboa 2012
N-ethyl N-hydroxyethyl aniline (NENHEA) as corrosion inhibitor for mild steel in 0.5 M sulfuric acid
N-etil N-hydroxietil anilina como inibidor de corrosão para o aço macio em ácido sulfúrico 0,5 M
Meenakshi Gupta1, Kalpana Bhrara1 and Gurmeet Singh1
1 Department of Chemistry, University of Delhi, Delhi-110007
Corresponding Author, e-mail: gurmeet123@yahoo.com
ABSTRACT
The efficiency of N-ethyl N-hydroxyethyl aniline (NENHEA) as corrosion inhibitor for mild steel in 0.5 M sulfuric acid has been investigated electrochemically by potentiodynamic and potentiostatic techniques, and surface morphology analysis by scanning electron microscopy. The results showed that the efficiency of NENHEA increases with the concentration and decreases with temperature. Corrosion potential indicate that this is an inhibitor of mixed type, although it is predominantly cathodic inhibitor. The adsorption of NENHEA on mild steel obeys Langmuir-Freundlich isotherm.
Keywords: Corrosion Inhibitor, Mild Steel, SEM, Langmuir-Freundlich Isotherm
RESUMO
A eficiência do inibidor de corrosão N-etil N-hidroxietil anilina (NENHEA) para o aço macio em 0,5M de ácido sulfúrico tem sido estudada por métodos electroquímicos através de técnicas potenciodinâmicas e potenciostáticas e por análise de superfícies recorrendo à microscopia electrónica de varrimento. Os resultados demonstram que a eficiência do NENHEA aumenta com a concentração e diminui com a temperatura. Os dados do potencial de corrosão indicam que este inibidor é do tipo misto, embora seja predominantemente do tipo catódico. A adsorção do NENHEA ao aço macio obedece ao modelo de isotérmicas Langmuir-Freundlich.
Palavras Chave: Inibidor de Corrosão, Aço Macio, MEV, Isótermica Langmuir-Freundlich
1. INTRODUCTION
Corrosion of metals is a major industrial problem that has attracted a lot of investigators in recent years [1-3]. Most of the efficient inhibitors used in industry are organic compounds that contain hetero-atoms (N, S, O) and/or multiple bonds in the molecules through which they are adsorbed onto the metal surface [4]. The existing literature shows that corrosion inhibition is a surface phenomenon which involves the adsorption of the organic compounds on metal surface [5, 6]. It has been observed that inhibition efficiency of organic compounds depends on electronic structure, molecular size, concentration of the molecules, surface morphology of the metal, the type of acid and interaction between metal and compound [7]. Large numbers of organic compounds with N as heteroatom, like amines, anilines, imines, imides have been studied as corrosion inhibitors [8-27]. In the present work, a study has been conducted on N-ethyl N-hydroxyethyl aniline (NENHEA, Molar Mass = 165.23 g mol-1) as corrosion inhibitor for mild steel in sulphuric acid. This compound has been investigated because it has heteroatoms like N and O [28], electron donating groups like ethyl, hydroxyl and p-electrons. Fig. 1 depicts the structure of the compound.
Figure 1
Structure of NENHEA
2. METHODOLOGIES
The corrosion of mild steel in the presence and absence of NENHEA has been investigated by potentiodynamic polarization, potentiostatic polarization and surface analysis.
2.1 Mild steel specimen
Experiments have been carried out using mild steel samples (C = 0.15%; Mn = 1.02%; Si = 0.025%; P = 0.025% and the remainder iron) with an exposed area of 1 cm2.
2.2 Potentiodynamic polarisation studies
Cathodic and anodic polarization studies have been performed on mild steel in 0.5 M H2SO4 in the presence of 10-1, 10-3, 10-5 and 10-7 M of NENHEA at 298, 308, 318 and 328 K. Three electrodes system was used, calomel electrode as reference electrode, platinum electrode as counter electrode and metal electrode as working electrode. The working electrode and reference electrode was immersed in a test solution for 4 hours until a steady state open circuit potential (OCP) was obtained. The current was passed to cell through the counter electrode and the cathodic polarization curve was obtained. Then after the regeneration of OCP, anodic polarization was obtained.
2.3 Potentiostatic polarization studies
Three electrodes system was used for potentiostatic polarization studies and the potential was applied through the cell at different values and the corresponding current values were noted. Polarization was carried out on mild steel in 0.5 M H2SO4 in the presence of 10-1, 10-3, 10-5 and 10-7 M NENHEA at 298 K.
2.4 Scanning electron microscopy (sem):
Polished cube of mild steel (1x1x1) cm was taken and washed with acetone and dried in dessicator. Mild steel coupons were dipped in 0.5 M H2SO4 in the presence of 10-1, 10-3, 10-5 and 10-7 M of NENHEA solution for 24 hours. They were then removed carefully without touching the surface, dried in a dessicator for 24 hours and then subjected to JEOL JSM 840 scanning electron microscope for SEM studies.
3. RESULTS
3.1 Potentiodynamic polarization studies
Figs. 2 a) to 2d) show the cathodic and anodic potentiodynamic curves obtained in 0.5 M H2SO4 for different concentration of NENHEA at several temperatures.
Figure 2
Potentiodynamic polarization curves for mild steel in 0.5 M H2SO4 with different concentrations of NENHEA at (a) 298, (b) 308, ( c) 318 and (d) 328 K.
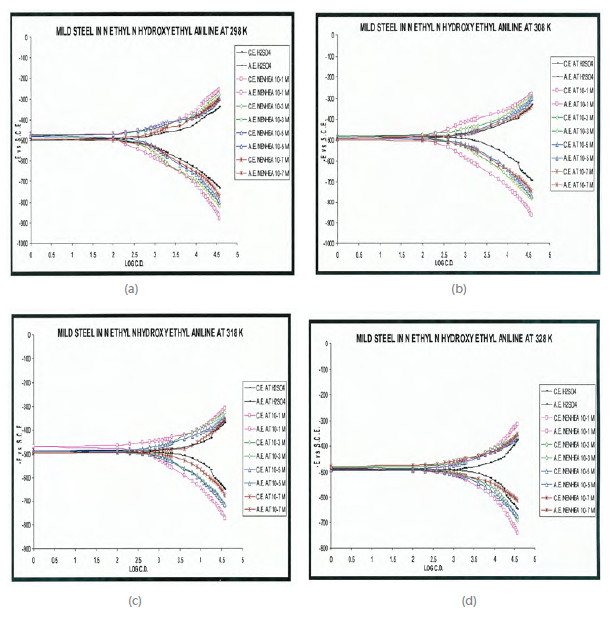
The corrosion current densities (icorr) were obtained by extrapolation of cathodic and anodic curves. Inhibition efficiency (I%) was calculated using Eqn. 1

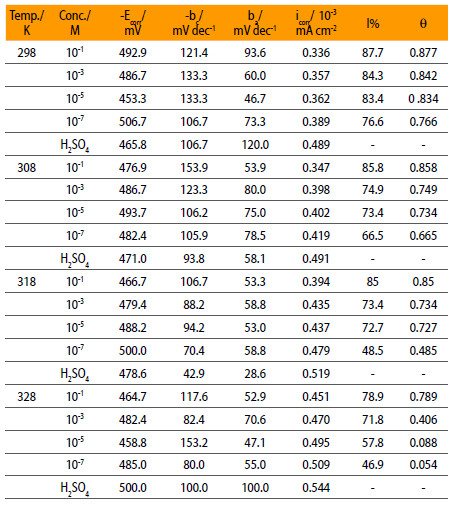
Where iin and io are, respectively, the corrosion current densities in inhibited and uninhibited solution. The values of various electrochemical parameters taken from the results such as the corrosion potential (Ecorr), cathodic Tafel slope (bc), anodic Tafel slope (ba), icorr and I% are summarized in Table 1.
Electrochemical parameters of mild steel in 0.5 M H2SO4 containing NENHEA.
3.2 Adsorption isotherm
The surface coverage (θ) is calculated using the equation,
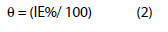
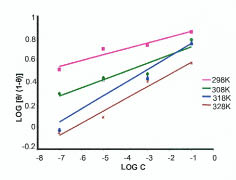
The adsorption behavior of the NENHEA on the metal surface was studied by fitting the data to various isotherms. Fig. 3 shows a straight line obtained on plotting log [θ/(1- θ)] vs. log c (with a slope which is not equal to unity) suggesting that the adsorption of NENHEA on mild steel in H2SO4 follows Langmuir-Freundlich isotherm (correlation coefficient R2= 0.9231).
Figure 3
Langmuir-Freundlich adsorption isotherms at different temperatures.
Langmuir-Freundlich adsorption isotherm is given by Eqn. 3
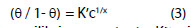
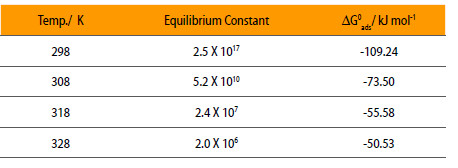
Where K is equilibrium constant = Kx, c is concentration, θ is surface coverage and x is number of inhibitor occupy in one active site. Using Eqn. 3, equilibrium constant, K is calculated at different temperatures and is given in Table 2.
Equilibrium Constant and standard free energy for the adsorption of mild steel in 0.5 M H2SO4 in the presence of NENHEA.
The free energy of adsorption at different temperatures is also calculated from the values of K using Eqn. 4
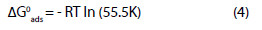
Eqn. 4 can also be written as

Rearrangement of Eqn. 5 shows that the plot of log K vs. 1/T should be a straight line. ΔH0ads and ΔS0ads can be calculated from the slope and intercept respectively. Fig. 4 shows the plot of log K vs. 1/T.
Figure 4
Plot of log K vs. 1/T
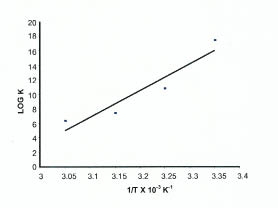
The values of ΔH0ads and ΔS0ads are found to be -701.55 kJ mol-1 and -2.01 kJ K-1 mol-1 respectively.
3.3 Effect of temperature
Eqn. 6 is used for calculating the energy of activation for the corrosion process.

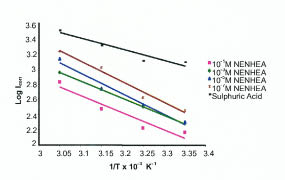
Where A is Arrhenius constant, Ea is activation energy, icorr is the corrosion current. Fig. 5 shows the plot of log icorr vs. 1/T and Table 3 gives the values of activation energy at different concentrations of NENHEA.
Energy of activation of mild steel in 0.5 M H2SO4 in the presence of NENHEA.
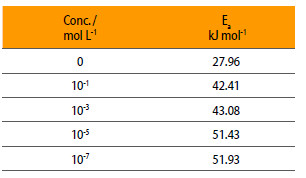
Figure 5
Plot of log icorr vs. 1/T for various concentrations of NENHEA.
3.4 Potentiostatic polarization studies
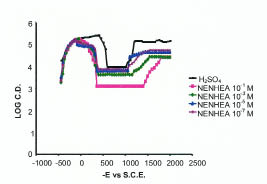
Passivity affects the mechanism of corrosion process because it produces a protective film that acts as a barrier to attack on the metal surface by the acidic environment. The effects of these additives have been studied in terms of electrochemical parameters, i.e., critical current (ic), primary passivation current (ipp), primary passivation potential (Epp) are shown in Fig. 6 and the corresponding data are given in Table 4.
Corrosion parameters of mild steel in 0.5 M H2SO4 in the presence of NENHEA.
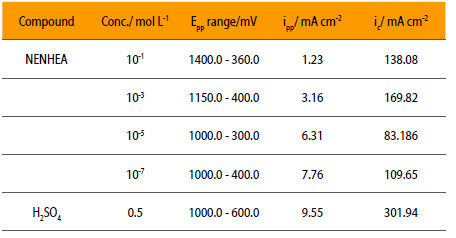
Figure 6
Potentiostatic polarization curve for mild steel in 0.5 M H2SO4 at various concentrations of NENHEA.
3.5 Scanning electron microscopy (sem)
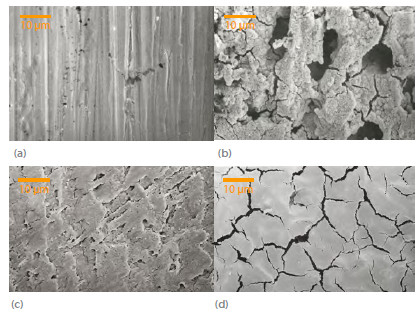
Fig. 7(a) shows scanning electron micrographs of plain mild steel whereas Fig. 7(b) shows the micrograph of mild steel in 0.5 mol L-1 sulphuric acid. Figs. 7 (c, d) are micrographs of mild steel in 0.5 M sulfuric acid in presence of 10-1 and 10-7 M NENHEA.
Figure 7
SEM of mild steel at 1000 magnification (a) Plain mild steel, (b) Mild steel in H2SO4, ( c) Mild steel in NENHEA (10-1 M) and (d) Mild steel in NENHEA (10-7M).
4. DISCUSSION
Table 1 shows that icorr at 298 K decreases from 0.489 x 10-3 mA/cm2 for the uninhibited solution to 0.336 x 10-3 mA/cm2 for the solution containing 10-1 M concentration of inhibitor, while this change is from 0.544 x 10-3 mA/cm2 to 0.451 x 10-3 mA/cm2 at 328 K.
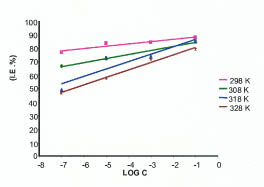
The inhibition efficiency is found to increase with increase in inhibitor concentration and decrease with increase in temperature as shown in Fig. 8.
Figure 8
Inhibition efficiency with inhibitor concentration at various temperatures.
With the increase in temperature the adsorption tends to weaken resulting in short time lag between adsorption and desorption thus leading to higher corrosion. The inhibition efficiency decreases with increase in temperature so; this additive may be physiosorbed on metal surface.
NENHEA does not show appreciable shift of Ecorr towards any direction that shows the mixed inhibitor behavior. Higher values of cathodic Tafel slopes bc) are obtained and this indicates that this inhibitor is cathodically more active i.e. reduces the hydrogen evolution reaction. NENHEA is mixed type inhibitor but acts predominantly as cathodic inhibitor. This additive show irregular trends in tafel slopes values due to different mechanism of adsorption on metal surface.
The negative values of ΔG0ads in Table 2 indicate the spontaneity of the adsorption of NENHEA on the surface of mild steel. The decrease in free energy of adsorption with increase in temperature suggests the physical adsorption of NENHEA on the surface of mild steel.
The negative value of ΔH0ads indicates that adsorption process is exothermic in nature whereas the negative value of ΔS0ads indicates that the adsorption of NENHEA on mild steel surface is an enthalpy favoured process.
The activation energy values (Table 3) of the inhibited system have been found to be higher than those of the uninhibited system, which indicates that the adsorption of NENHEA induces the energy barrier and makes the corrosion reactions more difficult to occur on the surface of mild steel.
Table 4 shows that the passivation in 0.5 M solution of sulphuric acid and 0,1 M NENHEA occurs in the range of potential between 360 and 1400 mV, while to the solution without inhibitor is 600 to 1000 mV. The value of iin decreases from 9.55 mA/cm2 for the uninhibited solution to 1.23 mA/cm2 for the solution containing 10-1M concentration of inhibitor, while this change is from 9.55 mA/cm2 to 7.76 mA/cm2 for the solution containing 10-7M concentration of inhibitor. Epp increases with the concentration of inhibitor conversely to ic that decreases.
This additive undergoes protonation to a considerable extent in H2SO4 [29], therefore the protonated species are not effective towards metal dissolution in active range, but becomes effective in passive range. The already adsorbed species like Fe(OH)ads may interact with those protonated species forming adsorbed layer on the surface and the complex of the type:
Fes[Fe(OH)3]-oxidelayer + InH+→ Fes[Fe-In-(OH)3]
Passivity may be due to the formation of [M-In-OH]ads or [M-OH-In]ads or [M-In-X]ads, where Xs are other anions present in the solution that may control the process. The irregular trends in Tafel slope values seem to confirm this hypothesis.
On comparing the surfaces of mild steel it turns out that the extent of corrosion decreases with increasing concentration of the inhibitor, in agreement with electrochemical results.
In synthesis we can say that NENHEA protects the mild steel surface in 0.5 M sulfuric acid by
· Neutralization of acid and making the medium less corrosive.
· Adsorption as well as by donating electrons from heteroatom like N and O.
· Protonated species adsorbed on the metal surface that reduce the hydrogen release.
CONCLUSIONS
N-Ethyl N-Hydroxylethyl aniline was used to protect the mild steel in 0.5 M sulfuric acid. The corrosion inhibition and adsorption characteristics have been studied by potentiodynamic and potentiostatic polarization at different concentrations and temperatures.
It can be concluded that:
1. NENHEA protects the mild steel in H2SO4 by getting adsorbed on the metal surface.
2. The corrosion inhibition efficiency increases with the concentration of NENHEA.
3. The inhibition efficiency decreases with temperature.
4. It is a mixed type of inhibitor but acts predominantly as cathodic inhibitor.
5. The adsorption of NENHEA on a mild steel surface obeys Langmuir-Freundlich isotherm.
6. NENHEA physiadsorbs on the mild steel surface in sulfuric acid since ΔG0ads=80 kJ/mol.
7. The values of Ea increases with the concentration of inhibitor.
8. SEM results agree well with electrochemical results.
REFERENCES
[1] G. Q. Liu, Z. Y. Zhu, W. Ke, C. I. Han and C. L. Zeng, Corrosion, 57, 730 (2001). [ Links ]
[2] W. D. Collins, R. E. Weyers and I. L. Al-Qadi, Corrosion, 49, 74 (1993). [ Links ]
[3] U. J. Ekpe, U. J. Ibok, B. I. Ita, O. E. Offiong and E. E. Ebenso, Mater. Chem. Phys., 40, 87 (1995). [ Links ]
[4] S. Tamilselvi and S. Rajeshwari, Anti-Corros. Methods and Mater., 50, 223 (2003). [ Links ]
[5] H. F. Finlay and N. Hackerman, J. Electrochem. Soc.,107, 259 (1961). [ Links ]
[6] E. E. Ebenso, P. C. Okafor, O. E. Offiong, B. I. Ita, U. J. Ibok and U. J. Ekpe, Bull. Electrochem., 17, 259 (2001). [ Links ]
[7] E. Stupnis EK- Lisac, S. Podbrseck and T. Soric, J. App. Electrochem., 24, 779 (1994). [ Links ]
[8] S. K. Rajappa and T. V. Ventakesha, J. Electrochem. Soc. India, 51, 54 (2002). [ Links ]
[9] J. M. Sykes, Br. Corros. J., 25, 175 (1990). [ Links ]
[10] A. El-Sayed, J. Appl. Electrochem., 27, 193 (1997). [ Links ]
[11] G. K. Gomma and M. H. Wahdan, Bull. Chem. Soc. Jpn., 67, 355 (1994). [ Links ]
[12] M. Elachowri, M. S. Hajji, S. Kertit, E. M. Essassi, M. Salem and R. Coudert, Corros. Sci., 37, 381 (1995). [ Links ]
[13] B. Mernari, H. Elattari, M. Traisnel, F. Bentiss and M. Lagrenee, Corros. Sci., 40, 391 (1998). [ Links ]
[14] N. Eldakar and K. Nobe, Corrosion, 33, 128 (1977). [ Links ]
[15] K. C. Emregül and O. Atakol, Mater. Chem. Phys., 83, 373 (2004). [ Links ]
[16] S. Sathiyanarayanan, C. Marikkannu and N. Palaniswamy, Appl. Surf. Sci., 241, 477 (2005). [ Links ]
[17] K. C. Emregül, A. A. Akay and O. Atakol, Mater. Chem. Phys., 93, 325 (2005). [ Links ]
[18] H. Ashassi-Sorkhabi, B. Shaabani and D. Seifzadeh, Appl. Surf. Sci., 239, 54 (2005). [ Links ]
[19] E. BayoL, K. Kayakirilmaz and M. Erbil, Mater. Chem. Phys., 104, 74 (2007). [ Links ]
[20] E. E. Oguzie, V.O. Njoku, C. K. Enenebeaku, C. O. Akalezi and C. Obi, Corros. Sci., 50, 3480 (2008). [ Links ]
[21] P. Singh, K. Bhrara and G. Singh, Appl. Surf. Sci., 254, 5927 (2008). [ Links ]
[22] M. R. Singh, K. Bhrara and G. Singh, Portug. Electrochim. Acta, 26, 479 (2008). [ Links ]
[23] I. Ahamad, R. Prasad and M. A. Quraishi, Mater. Chem. Phys., 124, 1155 (2010). [ Links ]
[24] Ramazan Solmaz, Corros. Sci., 52, 3321 (2010). [ Links ]
[25] A. Popova, M. Christov and A. Vasilev, Corros. Sci., 53, 1770 (2011). [ Links ]
[26] P. Volovitch, I. Gazizzullin, F. Ruel and K. Ogle, Corros. Sci., 53, 1362 (2011). [ Links ]
[27] S. Issaadi, T. Douadi, A. Zouaoui, S. Chafaa, M. A. Khan and G. Bouet, Corros. Sci., 53, 1484 (2011). [ Links ]
[28] I. Langmuir, J. Am. Chem. Soc., 38, 2263 (1916). [ Links ]
[29] G. P. Sharnin, I. F. Falyakhov and F. G. Khairutdinov, Chem. Heterocycl. Compd., 16, 1234 (1980). [ Links ]
Artigo submetido em Maio de 2011 e aceite Janeiro de 2012













