Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Corrosão e Protecção de Materiais
versão On-line ISSN 2182-6587
Corros. Prot. Mater. vol.31 no.1 Lisboa 2012
Hydrotesting and microbial corrosion
Testes hidrostáticos e corrosão microbiológica
C.A.C. Sequeira* 1, Luís P. S. Araújo2,
1 ICEMS-DEQB, Instituto Superior Técnico, Universidade Técnica de Lisboa, Avenida Rovisco Pais, 1049-001 Lisboa, Portugal
2 DEQ, FEUAN, Av. 21 de Janeiro, Luanda, Angola
Corresponding Author, e-mail: cesarsequeira@ist.utl.pt
ABSTRACT
The practice of hydrostatic testing in industry and its likelihood of microbial induced corrosion is discussed. It is recommended to avoid common pitfalls, to implement proper procedures, and to revise current standards.
Keywords: Hydrostatic Testing, Microbial Induced Corrosion, Biofilm Formation, Corrosion Inducing Bacteria, Biocides
RESUMO
O uso de testes hidrostáticos na indústria induz, em muitas situações, à corrosão microbiológica. Nessa medida, é importante evitar procedimentos menos correctos, nomeadamente rever as normas industriais correntes.
Palavras-chave: Testes Hidrostáticos, Corrosão Microbiológica, Formação de Biofilmes, Bactérias Indutoras de Corrosão, Biocidas
1. INTRODUCTION
Hydrotesting (HYD) is an industrial practice that is of frequent use in industry. The main characteristic of hydrotesting is that it is a leak and strength test. One important point of HYD is that, while it is of frequent use in different industries, its practice can actually render the tested system vulnerable to corrosion, especially microbiologically influenced corrosion (MIC) [1-4].
There are many factors that contribute to increasing the risk of MIC. For example, if we take a buried pipeline, the likelihood of MIC increases with factors such as: the choice of material, the type of coating and its condition, the environment around the pipe (pH, oxygen concentration), etc. If, for instance, the pipe is located in a waterlogged soil, the corrosion inducing bacteria (CIB) will have an increased chance of colonising the pipe and causing corrosion.
The risk of MIC will also increase if the following conditions arise:
1. The buried pipeline is located in a damp soil (thus having a fair population of microorganisms including CIB).
2. The material of the pipe is an ordinary carbon steel (note: numerous field examples and laboratory studies have shown that it is one of the materials most vulnerable to MIC).
3. There is no adequate cathodic protection (to partly prevent MIC).
4. The pipe is subject to poor HYD practice.
Figure 1 shows a scheme illustrating the inter-related factors (including HYD) affecting MIC.
Figure 1
Factors to be considered during hydrotesting (HYD) which affect the likelihood of the system being susceptible to MIC (adapted from [5]).
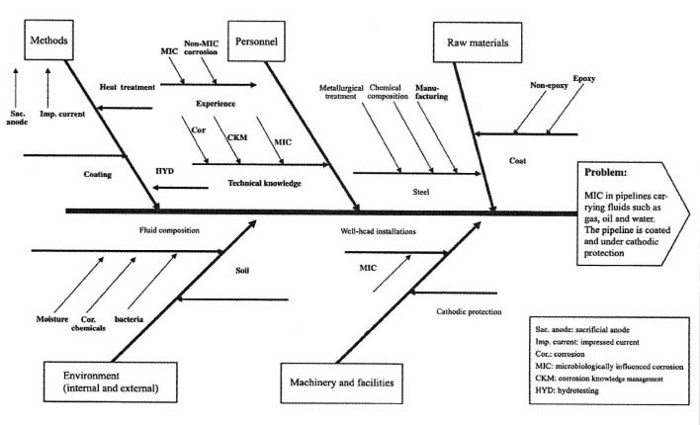
As indicated, one of the methods that can cause MIC is the practice of hydrotesting itself. While the exact mechanism of MIC resulting from HYD is not always known, two possible mechanisms may be considered and, in this respect, hydrotest practices can be classified into two categories: a wrong hydrotest where the water used as the hydrotesting medium is not treated, therefore it is a wrong water and incomplete hydrotest where the practice of draining and drying has not been carried out completely [5]. If the bacteria can find their way into the system by either wrong or incomplete hydrotesting, they can stay there and, literally, wait until the environmental conditions become favourable for their growth and activity. Where bacterial colonisation does take place, a local environment capable of producing localised corrosion (pitting) can develop.
Figure 2 presents an example of pitting arising from hydrotesting. Kobrin in late 1990s reviewed several cases of MIC that had occurred as a result of hydrotesting [6]. In this review, some of the common features of all the cases reported were:
Figure 2
Example of pitting induced during hydrotesting. The pitting varied from 1.5 to 2.0 mm in depth and was about 2-5 mm in diameter. Carbon steel in fresh water (adapted from [6]).
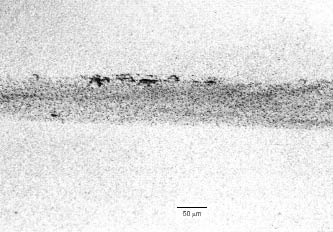
The water had not been drained after the hydrotest,
The water flow through the horizontal pipelines was low,
Water had been used as a ballast for settling purposes,
Water had been used for emergency purposes (such as fire extinguishing) with no provisions for continuous circulation.
While some alternatives to water for hydrotest media have been suggested and implemented [5], e.g. demineralised water or high-purity steam condensate, different combinations of water with other chemicals have also been applied. Some examples are, although not limited to [7], methanol/ fresh water, methanol/Kinetic Hydrate Inhibitors (KHIs), methanol/anti agglomerate low dosage hydrate inhbitors (AA LDHIs), brine-only solutions, e.g. KCl, NaCl or CaCl2 brine.
Although HYD is often critical for industries, the possible link between HYD and MIC is frequently ignored.
Of worldwide importance and practicality, especially in the oil and gas industry, are the so-called DNV (Det Norske Veritas) standards. The main standard which is consulted with regard to pressurizing and hydrotesting of off-shore pipes is DNV-OS-F101 (Oct.2007), Submarine Pipeline Systems. Section 6 and 10 of this standard are mainly related to the quality and required treatment of the fluid to be used for this purpose.
Examining the content of these standards shows us that the relevant items (relating to MIC) to be considered in the DNV standard are:
i. Item 0403 in Section 10 recommends that The water should be filtered to remove suspended particles larger than 50 µm.
ii. Guidance note of Section 6, D302, states that Use of freshwater should be considered or seawater treated to a pH of 9 minimum.
With regard to the likelihood of MIC, one apparent drawback in the standard (item i) is that by filtering, the probability that bacteria will be separated is extremely small. As previously noted, the average size of bacteria is about 1 µm and therefore, as such they can easily pass through a 50 µm filter. It is further assumed (item ii) that by limiting the food for bacteria or making the environment too alkaline, the possible impact of corrosion can be reduced.
DNV [8] are aware of such issues and recognise that: bacteria normally exist in fresh water (their activity being dependent on actual contents of dissolved salts and organic matter which can be controlled if fresh water is to be used for hydrotesting) and in seawater with a pH above 9. However, the bacterial growth is then much less intense and is not expected to cause any corrosion damage to the pipeline during the limited time of exposure to the hydrotest water (this is based on practical experience).
Whilst DNVs comments are taken on board the risk of MIC remains. The following section addresses some of the aspects which increase the risk of MIC.
2. BIOFILM FORMATION AND CORROSION
We will explain some mechanisms that, if ignored, can easily lead to a misunderstanding (myths) and consequent underestimation of the risk of MIC.
There are mainly two important concepts that need to be reviewed and understood:
1. Biofilm formation and the dynamic nature of its formation,
2. The ability of bacteria to resist environmental adverse conditions.
The dynamics of biofilm formation can be described by very simple stages, as illustrated in Figure 3.
Figure 3
implified schematic presentation showing biofilm formation and its dynamic nature:
1) bare metal, 2) initiation of biofilm formation, 3) increase in biofilm thickness, 4) detachment and 5) regrowth.
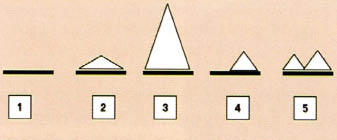
The biolfim is not a structure fixed in time and place: it is often continuously moving and it is alive. Biofilm formation evolves during several phases: Phase 1, the bare metal is itself exposed to the environment that contains CIB. The free-floating bacteria (planktonic bacteria as they are called) begin to settle on the surface of the metal and become relatively motionless or sessile. The main factors contributing to this change of phase from planktonic to sessile have been discussed elsewhere [9-11].
When the bacteria become settled, they start to build a structure that is known (wrongly!) as the biofilm. Most of the material in this structure is actually non-biological, hence bacteria make up a relatively low percentage of the dry-weight of the so-called biofilm. It is very difficult to say how much contact time is required to allow the attachment of the bacteria. It has been reported [9] that biofilm formation, depending upon the aqueous environment within which the metal has been immersed, may take minutes to hours. This uncertainty of the time required for bacterial attachment has implications for whether or not post hydrotest treatments should be carried out.
As the biofilm starts to grow and become thicker, it begins to act as a diffusion barrier to oxygen and nutrients. The implication of this is that some types of bacteria lying within the inner layers of the film may die. This, in turn, may lead to detachment via flow and mechanical impact that produces shear forces strong enough to overcome the adhesive forces developed by the biofilm. This phase is important because, whilst some detachment takes place, the whole biofilm will not be removed completely leaving a patchy network of biofilm, the extent of this network depending on physical, biological and chemical factors. The result is that this patchy structure can allow regrowth of new biofilm. The practical importance of this phase is that if biofilms are not removed completely, re-colonisation may occur on subsequent contact with an aqueous phase within in a relatively short time.
It is inferred in the standards that when the pH of the hydrotest environment becomes alkaline, the bacteria become inactive. This does not mean the bacteria are dead. Therefore, although a high pH can serve to make the bacteria inactive, as soon as bacteria are nested within the internal wall of the system (say a pipeline), they can go on fasting, thereby lowering their energy requirements through mechanisms such as reduction in size [12]. An important factor here is the possibility that spore-forming bacteria are introduced during hydrotesting. This type of bacteria, e.g. Clostridia, are capable of resisting many environmental adverse conditions. Therefore, making the environment highly alkaline or cutting the nutrients may not always work.
Another myth found in many industries is that sulphate reducing bacteria ( SRBs ) are the most important organism contributing to MIC [11-13,14]. This myth may have arisen from previously reported studies [11] which have cited the studies started with Hamiltons work [15], addressing MIC being wherein it is quoted that MIC is most commonly associated wit sulphate-reducing bacteria.
However, in addition to SRBs there are many other types of organisms (including bacteria) that can be effective on corrosion. These organisms can include fungi, algae and also other bacteria such as iron oxidising bacteria (IOB), ion reducing bacteria (IRB), sulphur oxidising bacteria (SOB), etc. Table 1 identifies some of these bacteria. More detailed data in this regard has been given elsewhere [16,17]. In addition, it is reported [18-20] that new strains of SRB with different mechanisms of electron transfer have been identified. This can certainly add complexity when considering the development of MIC by SRB highlighting that there are not only different types of CIB, but there is also diversity among SRB themselves. Under natural circumstances, it is highly probable that mixed cultures of bacteria will be found, of which SRB may be only a fraction. Some experimental studies using mixed cultures [21] have documented the corrosive effects of such environments.
Table 1
Examples of some corrosion-inducing bacteria.
Genus/species
Desulphovibrio
Desulphotomaculum
Desulphomonas
Acidithiobacillus thiooxidans
Acidithiobacillus ferrooxidans
Gallionella
Siderocapsa
Leptothrix
Sphaerotilus
Sphaerotilus natans
Pseudomonas
Pseudomonas aeruginosa
Another SRB related myth is that there is a relationship between the numbers of SRB and the rate of corrosion; the higher the number of SRB, the more severe the corrosion. Here, we see that the probable source of this prognosis is the work by Ronay et al. [22] where it is stated that if the number of SRB per gram is less than 5x103, there is no risk of MIC, whereas a count of 104 or more of SRB per gram of soil can lead to a severe case of MIC. Whilst this analysis has some value, it should only be adopted if we know to which bacteria we are applying it.
One of the ways by which CIB, such as SRB, can influence corrosion, is by affecting the electro-chemistry of the environment [23]. Therefore, theoretically, even a small population of bacteria can alter the chemistry of the system to render it corrosive. In this sense, this is in contrast to what bacteria such as sulphuric acid producing sulphur oxidising bacteria (SOB) do. It is obvious that the more SOB, the greater is the potential for more acid production leading to an increasingly corrosive environment. A study [24] has shown that microbial sulphate oxidation was occurring and that the rates of this activity could be enhanced by increasing the population of sulphur oxidising bacteria in the samples. While research shows that there is no relationship between the number of SRB and corrosion rate [25], these numbers can be useful when it comes to monitor the system. Two examples of such situations are (I) indicating the possibility of producing more sessile bacteria and (II) the effect of biocide application in a closed system containing stagnant water. Therefore, the reduction in the number of planktonic bacteria, measured by most quick tests, shows either they have been killed, they are no longer swimming around or they have been transformed to sessile bacteria [26].
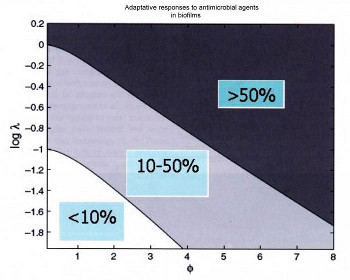
As seen from the above, there is not just one type of bacteria available in the water that can be used for HYD. Also, if the bacteria are capable of forming biofilm, this will prevent (or delay) the ingress of biocides deep into the biofilm. An example of such is shown in Figure 4 where as the biofilm becomes increasingly thicker, more bacteria start to become resistant to the biocide. This also emphasizes the fact that the biofilm monitoring is an essential mater and without an effective corrosion management program, that also considers the importance of MIC in place, just adopting the standards may not always guarantee prolonged lifetime, post hydrotesting.
Figure 4
The relation between dimensionless numbers representing biofilm thickness (f) and biocide action (log ?). Smaller ? shows thinner biofilms, whereas smaller ? values show faster acting biocide. The values in the squares in each region show the percentage of bacteria capable of becoming adaptive to the biocide (adapted from [24[]).
3. CONCLUSIONS
Hydrotesting is a practice routinely carried out in industry that has the potential to lead to corrosion-inducing bacteria. Hence, there is a serious need for all involved in either preparing standards or applying them with regard to the recognition that poor hydrotest practice may have important implications in the formation of MIC.
There appear to be certain misunderstandings and myths which lead to an underestimation of the impact of the presence of bacteria during hydrotesting and the subsequent increase in risk of MIC.
The current industry standards provide some advice on the necessary precautions required when conducting hydrotesting. However, these standards are somewhat generic and do not fully inform the user on the potential problem of MIC. It is recommended that such standards are revised.
REFERENCES
[1] C. A. C. Sequeira and A. K. Tiller, eds. (Microbial Corrosion – 1), Elsevier Applied Science, London, UK (1988). [ Links ]
[2] C. A. C. Sequeira and A. K. Tiller, eds. (Microbial Corrosion), European Federation of Corrosion Publications, The Institute of Materials, Number 8, London, UK (1992). [ Links ]
[3] A. K. Tiller and C. A. C. Sequeira, ed. (Microbial Corrosion) European Federation of Corrosion Publications, The Institute of Materials, Number 15, London, UK (1995). [ Links ]
[4] C. A. C. Sequeira, eds. (Microbial Corrosion), European Federation of Corrosion Publications, The Institute of Materials, Number 29, London, UK (2000). [ Links ]
[5] G. Stoecker (A Practical Manual on Microbiologically Influenced Corrosion) (G. Kobrin, ed.), NACE International, Houston, Texas, USA (1993). [ Links ]
[6] G. Kobrin, (Microbiologically influenced corrosion of stainless steel by water used for cooling and hydrostatic testing), NIDI Technical Series No. 10085, in Proceedings of 58th International Water Conference, November, Pittsburgh, PA, USA (1997). [ Links ]
[7] M. Erdogmus, L. Cowie, R. Chapman, G. Fung and P. Bollavaran (A novel approach to green, safe and economic subsea hydrotesting), CTC 17326, in Proceedings of Offshore Technology Conference, Houston, Texas, USA (2005). [ Links ]
[8] Private communication with the Section for Materials Technology and Failure Investigation, Det Norske Veritas, 29th October 2010. [ Links ]
[9] H. A. Videla (Mechanisms of MIC: Yesterday, today and tomorrow), in Proceedings of MIC – an International Perspective Symposium, Extrin Corrosion Consultants, Curtin University, Perth, Australia (2007). [ Links ]
[10] B. N. Herbert (Handbook of Biocide and Preservative Use), (H. W. Rossmoore, ed.), Blackie Academic & Professional, Chapman & Hall, Glasgow, UK (1995). [ Links ]
[11] A. Sanchez Del Junco, D. A. Moreno, C. Ranninger, J. J. Ortega-Calvo and C. Saiz-Jimenez, Int. Biodeter. Biodegr., 29, 367 (1992). [ Links ]
[12] C. C. Gaylard and H. A. Videla, eds. (Bioextraction and Biodeterioration of Metals), Cambridge University Press, Cambridge, UK (1995). [ Links ]
[13] F. Colin, M. J. Jourdain, A. Pourbaix and C. A. C. Sequeira (Functional simulation of bacterial corrosion at low enthalpy and application to the solution of corrosion problems), in Proceedings of the International Symposium on Geothermics 94 in Europe, BRGM, Vol. 230, 181, Orléans (1994). [ Links ]
[14] C. A. C. Sequeira (Microbiological and corrosive activity of sulphate reducing bacteria in low-enthalpy geothermal systems) in Proceedings of 45th Annual Meeting International Society of Electro-chemistry, Vol. 1, Porto, Portugal (1994). [ Links ]
[15] W. A. Hamilton, Annu. Rev. Microbiol., 39, 195 (1985). [ Links ]
[16] D. A. Jones and P. S. Amy, Corrosion, 58, 638 (2002). [ Links ]
[17] A. K. Lee, M. G. Buchler and D. K. Newman, Corros. Sci., 48, 165 (2006). [ Links ]
[18] W. R. Fischer, H. H. Paradies, A. H. L. Chamberlain and C. A. C. Sequeira (New types of corrosion impairing the reliability of copper in potable water caused by microorganisms), in Proceedings of 5th EC Conference on RTD on Industrial Technologies, p. 137, European Congress Consultants and Organisers, Brussels, Belgium (1994). [ Links ]
[19] C. A. C. Sequeira, A. C. P. R. P. Carrasco, D. Wagner, M. Tietz and W. R. Fischer (Membrane properties of biopolymeric substances), in Proceedings of the 3rd EFC Workshop on Microbial Corrosion, Estoril, March, 1994, EFC Publications Nº 15, The Institute of Materials, pp. 64, London, UK (1995). [ Links ]
[20] L. F. F. T. T. G. Rodrigues and C. A. C. Sequeira (Transport properties of natural biofilms developed in geothermal waters), in Proceedings of Electrochem. 95, University of Wales, Bangor, September, Society of Chemical Industry, London, UK (1995). [ Links ]
[21] R. A. Lane, The AMPTIAC Quarterly, 9, 3 (2005). [ Links ]
[22] D. Ronay, I. Fesus and A. Wolkober (New aspects in research in biocorrosion of underground structures), in Proccedings of Corrosion 87, Brighton, UK (1987). [ Links ]
[23] C. A. C. Sequeira (Recent Advances in Biodeterioration and Biodegradation) (K.L. Garg, N. Garg, K.E. Mukerji, eds.) Naya Prokash, Calcutta, Vol. II, pp. 117 (1994). [ Links ]
[24] P. A. Pryfogle (Monitoring Biological Activity at Geothermal Power Plants), Idaho National Laboratory, USA (2005). [ Links ]
[25] E. Ilhan-Sungur, N. Cansever and A. Cotuk, Corros. Sci., 49, 1097 (2007). [ Links ]
[26] B. Szomolay, I. Klapper, J. Dockery and P. S. Stewart, Environ.Microbiol., 7, 1186 (2005). [ Links ]
Artigo submetido em Setembro de 2011 e aceite em Janeiro de 2012













