Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciência e Técnica Vitivinícola
versão impressa ISSN 0254-0223
Ciência Téc. Vitiv. vol.26 no.2 Dois Portos 2011
Single-laboratory validation of determination of acetaldehyde, ethyl acetate, methanol and fusel alcohols in wine spirits, brandies and grape marc spirits using GC-FID
Ana Catarina P. M. N. Luís2, Deolinda Mota1, Ofélia Anjos2, Ilda Caldeira1,*
1 Instituto Nacional de Recursos Biológicos, I.P./INIA-Dois Portos. Quinta da Almoínha, 2565-191 Dois Portos. Portugal.
2Unidade Técnico Científica de Recursos Naturais e Desenvolvimento Sustentável, IPCB/ESA, Apartado 119, 6000 Castelo Branco, Portugal.
SUMMARY
This work presents the determination of principal volatile compounds (acetaldehyde, ethyl acetate, methanol, 2-butanol, 1-propanol, 2-methyl-1-propanol, 2-propen-1-ol, 1-butanol, 2-methyl-1-butanol and 3-methyl-1-butanol) in wine spirits and grape marc spirits by GC-FID. The method was evaluated in terms of suitability of chromatographic system, accuracy, precision, linearity and detection and quantification limits. The method showed good accuracy for the majority of the compounds and it presented linearity and precision for all compounds. Bases in these results the method seems suitable for quality control of marc spirits and wine spirits.
Key words: volatile compounds, validation, grape marc spirit, wine spirit
Validação interlaboratorial da determinação de acetaldeído, acetato de etilo, metanol e álcoois superiores em aguardentes vínicas, aguardentes vínicas envelhecidas e bagaceiras por GC-FID
RESUMO
Este trabalho apresenta a determinação dos principais compostos voláteis (acetaldeído, acetato de etilo, metanol, 2-butanol, 1-propanol, 2-metil-1-propanol, 2-propen-1-ol, 1-butanol, 2-metil-1-butanol e 3-metil-1-butanol) em aguardentes vínicas e bagaceiras por GC-FID. O método foi avaliado em termos de adequação do sistema cromatográfico, exactidão, precisão, linearidade e limites de detecção e quantificação. O método apresentou boa exactidão para a maioria dos compostos e apresentou linearidade e precisão para todos os compostos. Com base nestes resultados o método parece adequado para o controle de qualidade das aguardentes vínicas e bagaceiras.
Palavras-chave: compostos voláteis, validação, aguardente bagaceira, aguardente vínica
INTRODUCTION
The grape marc spirit called bagaceira is produced by distillation of fermented grape pomace while the wine spirit is produced from distillation of wine and the brandy is a wine spirit that stays in wooden barrels for a minimum of six months (Regulation EC n. 110/2008). These alcoholic beverages are complex mixtures mainly comprised of ethanol and water and a large number of minor volatile compounds such as alcohols, acids, esters and other compounds, that may be present in the raw materials (Quady and Guymon, 1973; Belchior and Carvalho, 1977, 1978; Bonnet, 1992; Boutinet et al., 1992; Mazerolles et al., 1992; Riponi et al., 1992;) or that could be formed during the manufacturing process (Belchior and Curvelo Garcia, 1971; Belchior and Carvalho, 1980; Carvalho and Belchior, 1983; Cantagrel et al., 1991; Galy et al., 1992; Segur and Bertrand, 1992; Silva and Malcata, 1999). Also the wood maturation period influence the volatile composition of the brandies (Guymon and Crowell, 1970; Onishi et al., 1977; Artajona, 1991; Rabier and Moutonet, 1991; Puech et al., 1992; Viriot et al., 1993; Guichard et al., 1995; Canas et al., 1999; Belchior et al., 2001; Canas, 2003; Caldeira et al., 2011).
The most abundant volatile compounds in these distilled spirits are the fusel alcohols, the fatty acid esters, together with acetaldehyde and methanol (Nykanen and Suomalainen, 1983; Nykanen, 1986; López-Vásquez et al., 2010). The fusel alcohols, ethyl acetate and the acetaldehyde are mainly resulting from yeast and bacteria metabolism during fermentation step (Bertrand and Suzuta, 1976; Nykänen, 1986; Nykänen and Nykänen, 1991) while the methanol is derived from enzymatic degradation of grape pectins (Gnekow and Ough, 1976).
The analysis of these volatile compounds is important to guarantee the quality control. It is important to control the levels of certain toxic substances, such as methanol and also it is necessary to guarantee the origin of the alcoholic beverages according to minimum levels (Regulation EC n. 110/2008) of volatile substances which comprise the higher alcohols, aldehydes and ethyl acetate (Regulation. EC n. 2870/2000). According to the regulatory requirements (CT83, 1990; Regulation EC n.2870/2000) these compounds must be determined by gas-liquid chromatography (GC) equipped with a flame ionization detector (FID).
Thus, this paper reports the single laboratory validation of a GC-FID method for the quantification of methanol, acetaldehyde, ethyl acetate and higher alcohols in wine spirits, brandies and grape marc spirits.
MATERIAL AND METHODS
Method validation
System suitability – according to the guidelines for chromatographic methods (CDER, 1994) it was evaluated the capacity factor k´, the relative retention ( α), the peak resolution (Rs), the tailing factor (T), theoretical number of plates (N) and injection repeatability:
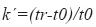
where t0=elution time of the void volume or non- retained components and tr=retention time of the compound
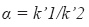
where k´1 = capacity factor of compound 1 and k´2 = capacity factor of compound 2
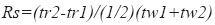
where tr2 and tr1= retention time respectively of compound 2 and compound 1 and tw1 and tw2=peak width respectively of compound 1and compound 2 measured at baseline of the extrapolated straight sides to baseline
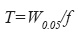
where W0.05=width of peak at 5% of the height and f=distance between peak maximum and peak front at W0.05
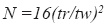
where tr= retention time and tw =peak width measured at baseline of the extrapolated straight sides to baseline
The injection repeatability was evaluated by the calculation relative standard deviation (RSD) of ten injections of different samples.
Calibration, linearity and range – standard solutions containing known amounts of the volatile compounds were analysed by the GC-FID procedure. Seven concentration levels, including the zero were analysed. The range of concentrations tested can be seen in Table I. Three injections were done for each sample or standard solution. The relative area was plotted against the relative concentration. For all the compounds the linear model is adjusted by the least-squares method. The choice of the model was based on the following statistical criteria: the slope is significantly different from zero; correlation coefficient superior to 0.99; the intercept is not significantly different from zero; the evaluation of residual residues (Bouvier, 1994).
Accuracy-recovery data - A grape marc spirit was spiked with known amounts of the studied volatile compounds at four different levels. The sample, at each level, was analysed by the proposed method in triplicate (OIV, 2011).
Precision - analysis repeatability - the repeatability was calculated by the analysis of the results of several replicas of grape marc spirits and brandies, according to the procedure described by OIV (2005, 2011). According to this procedure the repeatability (r) was calculated according to the formula:
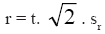
in which:
t = value of t-Student distribution
Sr = the repeatability standard deviation calculated according to the expression:
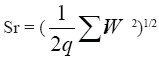
where
q = the number of samples analyzed in duplicate
Wi = the absolute differences between duplicates
Detection and quantification limits – Detection limit (DL), the lowest analyte amount that can be detected, and quantification limit (QL), the lowest analyte amount that can be quantified with acceptable precision, were estimated by the graph approach, by analysing the ground noise of three chromatograms of an analytic blank (hydroalcoolic solution 50% v/v) (OIV, 2011).
Reagents
Ethanol was purchased from Merck (Darmstadt, Germany). Distilled water was used to prepare the hydroalcoholic solutions.
GC-FID standards: Ethyl acetate [CAS Nº 141-78-6; purity ≥99.8%] was purchased from Riedel-de-Haen (Seelze, Germany), methanol [CAS Nº 67-56-1; purity ≥99.9%] was purchased from Merck (Darmstadt, Germany). 2-methyl-1-butanol [CAS Nº 34713-94-5; purity ≥98%] 3-methyl-1-butanol [CAS Nº 123-51-3; purity ≥98.5%], 1-butanol [CAS Nº 71-36-3; purity ≥99.5%], 2-methyl-1-propanol [CAS Nº 78-83-1; purity ≥99.5%], 1-propanol [CAS Nº 71-23-8; purity ≥99.5%], 2-propen-1-ol [CAS Nº 107-18-6; purity ≥98%], 2-butanol [CAS Nº 78-92-2; purity ≥99.5%], 4-methyl-2-pentanol [CAS Nº 108-11-2; purity ≥97%] and acetaldehyde . [CAS Nº 75-07-0; purity ≥99.5%] were purchased from Fluka (Buchs, Switzerland).
Preparation of standard solutions
All standard solutions were prepared in a 50% (v/v) hydroalcoholic solution by using concentration ranges expected for each distilled beverage, in a laboratory room maintained at 20º C.
a) Solution A – approximately 500 mg of acetaldehyde, 1000 mg of ethyl acetate and 5000 mg of methanol were weighted into a 100 mL volumetric flask containing approximately 10 ml of hydroalcoholic solution of 50% in order to minimise evaporation. After, the volumetric flask was filled with hydroalcoholic solution of 50%. The exact weight of each reagent was recorded in order to perform the calculations.
b) Solution B – approximately 50 mg of 2-butanol, 300 mg of 1-propanol, 500mg of methyl-1-propanol, 50mg of 2-propen-1-ol, 50 mg of 1-butanol, 500 mg of methyl-2-butanol, and 1250mg of 3-methyl-1-butanol were weighted into a 100 mL volumetric flask containing approximately 10 ml of hydroalcoholic solution of 50% in order to minimise evaporation. After ,the volumetric flask was filled with hydroalcoholic solution of 50% and the exact weight of each reagent was recorded in order to perform the calculations.
c) Solution C – approximately 550 mg of 4.methyl-2-pentanol (internal standard) was weighted into a 100 mL volumetric flask containing approximately 10 ml of hydroalcoholic solution of 50% in order to minimise evaporation. After, the volumetric flask was filled with hydroalcoholic solution of 50% and the exact weight of the reagent was recorded in order to perform the calculations.
d) Standard solutions for the evaluation of linearity – Aliquots of different volumes of solution A and B were taken into a 100 ml of volumetric flasks, which were filled with hydroalcoholic solution of 50% v/v.
Alcohol strength
Alcohol strength was determined by distillation followed by the determination of alcohol content of distillate using an electronic densimeter (DMA5000, Antoon Paar) [Regulation EC n. 2870/2000]. The results are present as volumetric percentage of ethanol in the beverage.
Preparation of samples to GC-FID
The compounds, in the spirit drinks, are determined by direct injection of the spirit drink or by the injection of the distillate, obtained in the alcohol strength determination, for the wooden aged spirit drinks. Prior to injection 9 ml of each sample (spirit drink, distillate of spirit drink, standard solution) was added with 1 ml of internal standard solution (solution C). The mixture was shaken and about 1 µl was injected in the gas chromatograph. The concentration of each compound is determined with respect to the internal standard from response factors, which are obtained during calibration using the standard solutions. The concentration of the compounds are expressed as mg/L or as g/100L of absolute ethanol (using the alcohol strength results) in order to verify the regulatory requirements (Regulation EC 2870/2000).
Gas chromatography-flame ionization detection (GC-FID)
GC-FID analysis was carried out using an Focus GC gas chromatograph (Thermo Scientific, USA) equipped with a flame ionisation detector-FID (250ºC) and a fused silica capillary column of polyethylene glycol (DB-WAX, JW Scientific, Folsom, CA, USA), 60 m length, 0.32 mm i.d., 0.25 µm film thickness. The carrier gas was hydrogen (3,40 cm3.min-1). The samples were injected (~1 µL) on the injector (200 ºC) in split mode (split ratio 1:6). The oven temperature program was 35 ºC (for 8 min), then increased at 10ºC.min-1 to 200 ºC and held for this temperature for a further 9 min.
Statistical analysis: Regression analysis and analysis of variance were performed. All calculations were carried out using Statistica vs. 98 edition (Statsoft Inc., Tulsa, USA).
RESULTS AND DISCUSSION
Chomatographic system suitability
The specificity of the method was evaluated by comparing retention times obtained for standard compounds mixtures with those obtained for alcoholic beverages. The specificity was evaluated by analysing the peaks for the purity and resolution from the nearest peak (Table I). Chromatograms of the standard solutions, brandy samples and grape marc spirits samples are shown in Figure 1.
Chromatographic system data: retention times, capacity factors, relative retention, resolution, tailing factors and theoretical number of plates
Dados do sistema cromatográfico: tempos de retenção, factores de capacidade, retenção relativa, resolução, factores de simetria, número de pratos teóricos
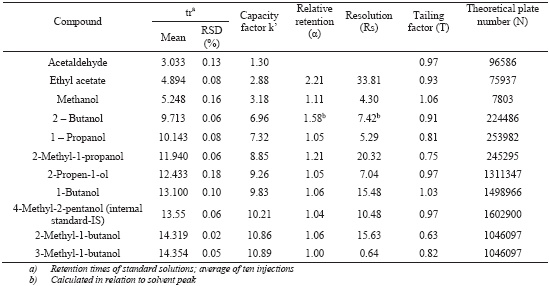
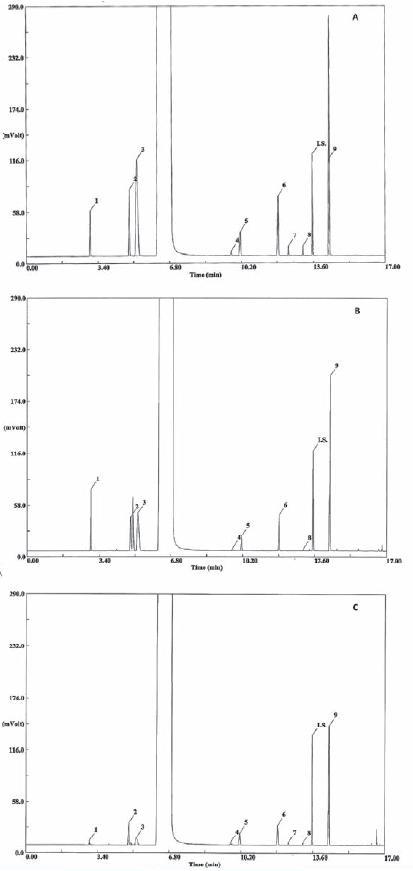
Fig. 1 - Chromatogram of hydroalcoolic solution of standards (A), of a grape mark spirit (B) and of a brandy (C). Compound identification: 1:acetaldehyde, 2:ethyl acetate, 3:methanol, 4:2-butanol; 5:1-propanol; 6:2-methyl-1-propanol, 7:2-propen-1-ol; 8:1-butanol; IS:4-methyl-2-pentanol (internal standard); 9:2-methyl-1-butanol+3-methyl-1-butanol
Cromatograma de uma solução hidroalcólica de padrões (A), de uma aguardente bagaceira (B) e de uma aguardente vínica envelhecida (C). Identificação dos compostos:1:acetaldeído, 2:acetato de etilo, 3:metanol, 4:2-butanol; 5:1-propanol; 6:2-metil-1-propanol, 7:2-propeno-1-ol; 8:1-butanol; IS:4-metil-2-pentanol (padrão interno); 9:2-metil-1-butanol+3-metil-1-butanol
Retention times, peak shape and peak resolution reveal that no interferences seem to be present in the chromatographic region of interest, where the analytes are located. All the peaks (Table I) had a resolution (Rs) higher than 2 with exception of 3-methyl-1-butanol. However, Kelly et al. (1999) showed that the 2-methyl-1-butanol and 3-methyl -1-butanol could be quantified individually or like one peak with similar results. Concerning the tailing factor, which affects the accuracy of quantitation, all the peaks presents a value below 2, according to the recommendations of CDER (1994). For all the compounds the theoretical number of plates is above the recommended 2000 (CDER, 1994).
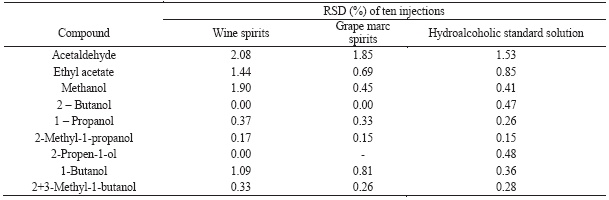
The precision of chromatographic system was evaluated by calculation of relative standard deviation (RSD) of multiple injections of different samples (hydroalcoholic, wine spirit and grape marc spirit) and the results are presented at Table II. For the majority of the compounds the RSD was lower than 1% and it range from 0.41 until 2.08 for methanol, ethyl acetate and acetaldehyde.
TABLE II
Injection repeatability evaluated by the relative standard deviation of ten injections of different samples (standard solution, wine spirits and grape marc spirits).
Repetibilidade de injecção avaliada pelo coeficiente de variação de dez injecções de diferentes amostras (solução padrão, aguardente vínica).
Linearity
The calibration graphs (relative area versus relative concentration) were built for each compound. The linear method is adjusted by least-squares method and the choice of the linear method was based on statistical criteria: the slope is significantly different from zero and correlation coefficient >0.99.
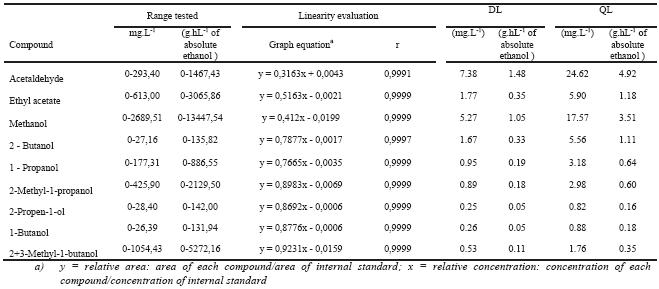
Table III summarises the linearity evaluation of the method for all the compounds.
The coefficient of correlation ranges from 0.9991 until 0.9999. As can be seen in Table III, all the compounds showed good linearity in the studied range with regression coefficients higher than 0.999.
TABLE III
Method linearity data and detection and quantification limits.
Resultados da linearidade do método e limites de detecção e quantificação
The observation of residual plots also confirms the linearity results.
The detection limits ranged from 0.25 until 7.38 mg.L-1 (Table III). The higher values are observed in some compounds which presents higher levels in the wine and grape marc spirits, such as methanol, ethyl acetate and acetaldehyde.
Accuracy-Recovery data
The recovery data, calculated by spiking a sample of grape marc spirit with four increasing known amounts of standards, are presented at Table IV.
TABLE IV
Recovery data obtained in spiked samples of a grape marc spirit
Resultados da percentagem de recuperação avaliada sobre uma amostra de aguardente bagaceira
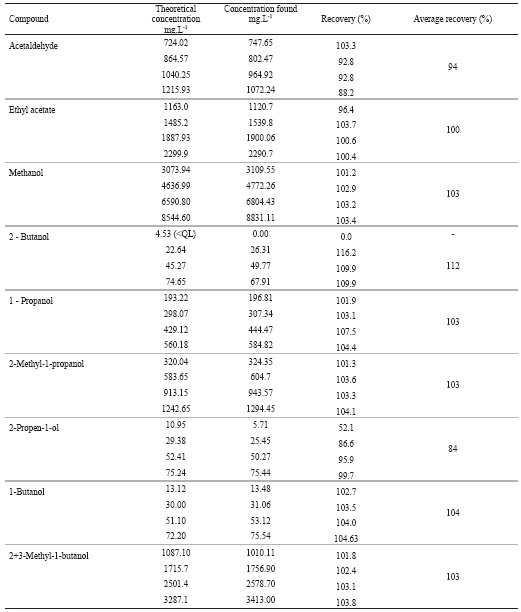
The recover data are acceptable for the majority of the compounds with an average recovery between 100 and 112%. Similar results are verified in cider spirits and Turkish raki (Madrera and Valles, 2007; Yilmaztekin and Cabaroglu, 2011). However the results for acetaldehyde and 2-propen-1ol suggest a matrix effect because the recovery data are depending of the added amount. Yilmaztekin and Cabaroglu, (2011) found better accuracy results in the determination of acetaldehyde in Turkish raki, but the range evaluated was lower and the recovery assays were done in standard solutions. So, future recovery assays must be done with these two compounds.
Precision – analysis repeatibility
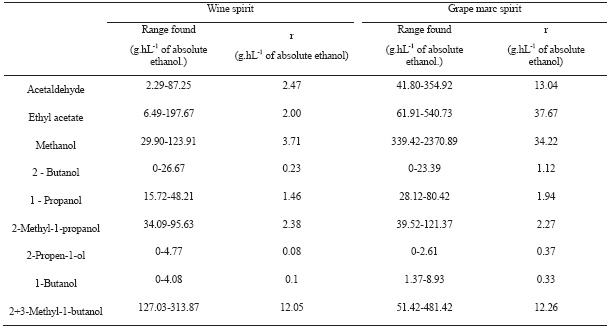
The repeatability (r) of the method was calculated by the analysis of several samples and its replicas obtained in repeatable conditions (same operator, same equipment and same laboratory). The value r means that in 95% of the cases, the difference between two values acquired under repeatable conditions will be lower than or equal to r.
The values obtained with a sample set of wine spirits (38 samples) and grape mark spirits (48 samples) are presented at Table V. Similars results were found by Duarte (2005). For acetaldehyde, ethyl acetate and methanol the repeatability (r) was higher on grape mark spirit than on the wine spirits, but also the compounds are presented at higher levels in the mark spirits.
TABLE V
Repeatibility values (r) determined in wine and grape marc spirits
Valores de repetibilidade (r) determinados em aguardentes vínicas e aguardentes bagaceiras
CONCLUSIONS
The proposed method presented linearity and precision for all compounds, as well as good recoveries for the majority of the compounds. These results show that the method is suitable for quality control of wine and grape mark spirits.
REFERENCES
Artajona J., 1991. Caracterisation del roble según su origen y grado de tostado, mediante la utilizacion de GC y HPLC. Viticultura/Enologia Profesional, 14, 61-72. [ Links ]
Belchior A.P, Caldeira I., Costa S., Lopes C., Tralhão G., Ferrão A.F.M., Mateus Ana M., 2001. Evolução das características físico-químicas e organolépticas de aguardentes Lourinhã ao longo de cinco anos de envelhecimento em madeiras de carvalho e castanheiro. Ciência Tec. Vitiv., 16, 81-94. [ Links ]
Belchior A.P., Carvalho E., 1977. Qualidade e composição química das aguardentes de bagaço. Influência dos alambiques Déroy e Caldeira Bagaceira. Vin. Port. Doc., 7, 9-15. [ Links ]
Belchior A.P., Carvalho E., 1978. Implicações da termovinificação nos teores em metanol e nas fermentações dos bagaços. Vin. Port. Doc., 8, 1-14. [ Links ]
Belchior A.P., Carvalho E., 1980. Factores que condicionam os teores de metanol nos bagaços. Vin. Port. Doc., 10, 1-9. [ Links ]
Belchior A.P., Curvelo Garcia A.S. 1971. Comportamento de alguns constituintes voláteis das aguardentes tipo Cognac no decurso da destilação. Vin. Port. Doc., 6, 1-14. [ Links ]
Bertrand A., Suzuta A., 1976. Formation of 2-butanol by lactic acid bacteria isolated from wine. Conn. Vigne Vin, 10, 409-426. [ Links ]
Bonnet J., 1992. Évolution du pressurage en Charentes en vue de l´amélioration qualitative des eaux-de-vie de Cognac. In: Élaboration et connaissance des spiritueux. 205-207. Cantagrel R. (ed.), TEC & DOC-Lavoisier, Cognac. [ Links ]
Boutinet S., Lurton L., Galy B., Mazerolles G., Gaschet J., 1992. Cinétiques de formation des prioncipaux constituants des eaux-de-vie nouvelles au cours de la fermentation alcoolique. In: Elaboration et connaissance des spiritueux. 213-217. Cantagrel R. (ed.), TEC & DOC-Lavoisier, Cognac. [ Links ]
Bouvier J.C., 1994. Guide d`évaluation et de la validation d`une méthode d`analyse chimique. 239p. INRA. [ Links ]
Caldeira I, Bruno de Sousa R., Belchior A.P., Clímaco M.C., 2011. Odorant Compounds of Aged Wine Brandies: The Wooden Barrel Role In: The Biology of Odors: Sources, Olfaction and Response 131-152, Weiss L.E. ; Atwood J.M. (eds.), Nova Science Publishers, Inc., New York. [ Links ]
Canas S., 2003. Estudo dos compostos extraíveis de madeira (Carvalho e Castanheiro) e dos processos de extracção na perspectiva do envelhecimento em Enologia. 303 p. Tese de Doutouramento em Engenharia Agro-Industrial, UTL-ISA, Lisboa. [ Links ]
Canas S., Leandro M.C., Spranger M.I., Belchior A.P.,1999. Low molecular weight organic compounds of chestnut wood (Castanea sativa L.) and corresponding aged brandies. J. Agric. Food Chem., 47, 5023-5030. [ Links ]
Cantagrel R., Lurton L., Vidal J.P., Galy B., 1991. La distillation Charentaise pour l' obtention des eaux-de-vie de Cognac. In: Les eaux de vie traditionelles d`origine viticole. 60-69. Bertrand A. (ed.), Lavoisier-TEC & DOC, Paris. [ Links ]
Carvalho E.C., Belchior A.P., 1983. Influência do aquecimento do alambique "Charentais" no rendimento e qualidade da aguardente. Ciência Téc. Vitiv., 2, 67-72. [ Links ]
CDER-Center for Drug Evaluation and Research, 1994. Reviewer Guidance' Validation of Chromatographic Methods 30p. [ Links ]
CT83, 1990. NP3263, Bebidas alcoólicas e espirituosas - determinação dos teores de etanal, acetato de etilo, metanol, 2-butanol, 1-propanol, 2-metil-1-propanol, 2-propeno-1-ol, 1-butanol, 2-metil-1-butanol+3-metil-1-butanol. IQA, Lisboa. [ Links ]
Duarte L.I.R., 2005 Validação do método de determinação dos teores de álcoois superiores, etanal, metanol e acetato de etilo por cromatografia gasosa. 99p. Trabalho de fim de curso em Engenharia Alimentar. ESAS-IPS, Santarém. [ Links ]
Galy B., Roulland C., Lurton L., Cantagrel R., 1992. Connaissance des paramètres influant sur la conservation des vins destinés à l´élaboration des eaux-de-vie de Cognac. In: Élaboration et connaissance des spiritueux. 218-224. Cantagrel R. (ed.), TEC & DOC-Lavoisier, Cognac. [ Links ]
Gnekow B., Ough C.S. 1976 Methanol in Wines and Musts: Source and Amounts Am. J. Enol. Vitic. 27, 1-6. [ Links ]
Guichard E., Fournier N., Masson G., Puech J.-L. 1995. Stereoisomers of β-methyl-γ-octalactone. I-Quantification in brandies as a function of wood origin and treatment of the barrels. Am. J. Enol. Vitic., 46, 419-423. [ Links ]
Guymon J.F., Crowell E.A., 1970. Brandy aging. Some comparisons of American and French oak cooperage. Wines & Vines, 1, 23-25. [ Links ]
Kelly J, Chapman S, Brereton P, Bertrand A, Guillou C, Wittkowski R. 1999 Gas chromatographic determination of volatile congeners in spirit drinks: interlaboratory study. J AOAC Int.;82, 1375-88. [ Links ]
López-Vásquez C., Bollaín M.H., Berstch K., Orriols I., 2010 Fast determination of principal volatile compounds in distilled spirits. Food Control, 21, 1436-1441. [ Links ]
Madrera R.R., Valles B.S., 2007. Determination of volatile compounds in cider spirits by gas chromatography with direct injection. J. Chromat. Sci., 45, 428-434. [ Links ]
Mazerolles G., Vidal J.P., Lablanquie O., Cantagrel R., 1992. Caractérisation analytique des eaux-de-vie nouvelles provenant de récoltes différentes. In: Élaboration et connaissance des spiritueux. 428-431. Cantagrel R. (ed.), TEC & DOC-Lavoisier, Cognac. [ Links ]
Nykänen L., 1986. Formation and occurrence of flavor compounds in wine and distilled alcoholic beverages. Am. J. Enol. Vitic., 37, 84-96. [ Links ]
Nykänen L., Nykänen I., 1991. Distilled beverages. In: Volatile compounds in food and beverages. 547-580. Maarse H. (ed.), Marcell Dekker Inc., New York. [ Links ]
Nykänen L., Suomalainen H., 1983. Aroma of beer, wine and distilled alcoholic beverages. 413p. D. Reidel Publishing Company, Dordrecht, Holland. [ Links ]
OIV, 2005 Compendium of international methods of wine and must analysis. Annex E – Laboartory quality assurance. Paris. [ Links ]
OIV, 2011 Compendium of international methods of wine and must analysis. Annex E – Laboartory quality assurance. Paris. [ Links ]
Onishi M., Guymon J.F., Crowell E.A., 1977. Changes in some volatile constituents of brandy during aging. Am. J. Enol. Vitic., 28, 152-158. [ Links ]
Puech J.-L., Lepoutre J.P., Baumes R., Bayonove C., Moutounet M., 1992. Influence du thermotraitement des barriques sur l´évolution de quelques composants issus du bois de chêne dans les eaux-de-vie. In: Elaboration et connaissance des spiritueux. 583-588. Cantagrel R. (ed.), TEC & DOC-Lavoisier e BNIC, Cognac. [ Links ]
Quady A.K., Guymon J.F., 1973. Relation of maturity, acidity, and growing region of 'Thompson Seedless' and 'Franch Colombard' grapes tom wine aroma and quality of brandy distillate. Am. J. Enol. Vitic., 24, 166-175. [ Links ]
Rabier Ph., Moutounet M., 1991. Évolution d`extractibles de bois de chêne dans une eau-de-vie de vin. Incidence du thermotraitement des barriques. In: Les eaux de vie traditionelles d`origine viticole. 220-230. Bertrand A., TEC & DOC-Lavoisier, Paris. [ Links ]
Regulation EC N. 110/2008 of the European parliament and of the council of 15 January 2008 on the definition, description, presentation, labelling and the protection of geographical indications of spirit drinks and repealing Council Regulation (EEC) No 1576/89 Off. J. Eur. Commun. L39, 16–54. [ Links ]
Regulation EC N. 2870/2000 of 19 December 2000 laying down Community reference methods for the analysis of spirits drinks. Off. J. Eur. Commun. L333, 20–46. [ Links ]
Riponi C., Antonelli A., Carnacini A., Motta M., 1992. Aptitudes de certaines souches de levures à l´elaboration de vins pour la production d ´eaux de vie. In: Elaboration et connaissance des spiritueux. 161-171. Cantagrel R. (ed.), TEC & DOC-Lavoisier, Cognac. [ Links ]
Segur M.C., Bertrand A., 1992. La distillation continue armagnacaise. In: Elaboration et connaissance des spiritueux. 257-266. Cantagrel R. (ed.), TEC & DOC-Lavoisier, Cognac [ Links ]
Silva M.L., Malcata F. X., 1999. Effects of time of grape pomace fermentation the chemical composition of grape marcs Z Lebensm Unters Forsch A 208: 134–143. [ Links ]
Viriot C., Scalbert A., Lapierre C., Moutounet M., 1993. Ellagitannins and lignins in aging of spirits in oak barrels. J. Agric. Food Chem., 41, 1872-1879. [ Links ]
Yilmaztekin M., Cabaroglu T. 2011 Confirmatory method for the determining of volatile congeners and methanol in Turkish Raki according to Europena Union Regulation (EEC) Nº 2000R2870: Single laboratory validation. J. AOAC Int., 94, 611-617. [ Links ]
ACKNOWLEDGMENTS
The authors knowledge the technical support of Amélia Soares and Otília Cerveira.
(Manuscrito recebido em 18.10.11. Aceite para publicação em 09.12.11)
*Corresponding author: Ilda Caldeira. Instituto Nacional de Recursos Biológicos, I.P./INIA-Dois Portos. Quinta da Almoínha, 2565-191 Dois Portos. Portugal. Tel.:+351261712106, Email: ilda.caldeira@inrb.pt













