Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta v.28 n.2 Coimbra 2010
Adsorption and Inhibitive Properties of Lincomycin for the Corrosion of Zinc in 0.01 – 0.05 M H2SO4
N.O. Eddy,1,* S.A. Odoemelam,2 E.C. Ogoko,2 B.I. Ita3
1 Department of Chemistry, Ahmadu Bello University, Zaria, Nigeria
2 Department of Chemistry, Michael Okpara University of Agriculture, Umudike, Nigeria
3 Department of Chemistry, University of Calabar, Calabar, Nigeria
DOI: 10.4152/pea.201002073
Abstract
Inhibition of the corrosion of zinc in various concentrations of H2SO4 by lincomycin was studied using weight loss and hydrogen evolution methods. The results obtained indicated that lincomycin is a good adsorption inhibitor for the corrosion of zinc in H2SO4 solutions. The inhibition efficiencies of lincomycin for the corrosion of zinc in H2SO4 were found to range from 70.90 to 80.32 %, 69.25 to 77.70 % and from 52.11 to 67.49 % at 303, 313 and 323 K, respectively. The inhibition efficiencies decreased with increase in temperature and with increasing concentration of H2SO4 but increased with increase in the concentration of lincomycin. The adsorption of lincomycin on Zn surface is endothermic, spontaneous and is best described by Langmuir adsorption isotherm. Base on the trend in the variation of inhibition efficiency with temperature and the calculated values of the activation and free energies of adsorption, a physical adsorption mechanism is proposed for the adsorption of lincomycin on the surface of zinc.
Keywords: corrosion, inhibition, Zn, lincomycin.
Introduction
Zinc is an important metal with numerous industrial applications and is mainly used for the corrosion protection of steel [1-3]. The zinc-coated steel materials provide a greater resistance to corrosion, but when exposed to humid atmosphere, they undergo rapid corrosion with the formation of a corrosion product known as white rust.
The study of corrosion of zinc and its inhibition is a subject of practical significance. For scale removal and cleaning of zinc surfaces with acidic solutions, the use of inhibitors is necessary. Many researchers in the literature studied the inhibition of Zn corrosion in HCl using organic compounds containing nitrogen, oxygen and/or sulphur atoms. Some nitrogen containing compounds such as thiourea, schiff base, hydrazine derivatives, aniline, ephedrine, narcotine, brucine and stryctuine have been reported to be good inhibitors for the corrosion of zinc [4-5]. Also, compounds such as quinine sulphate, piperazine, caffine, barbitone, amide, and pyridine derivatives have been investigated as corrosion inhibitors for zinc in acidic medium [6]. In spite of these developments, there are very few inhibitors that are effective for the inhibition of the corrosion of zinc in H2SO4. Besides, most of the good inhibitors are toxic because they contain heavy metals or other toxic compounds.
A close examination of various corrosion inhibitors revealed that the choice of the corrosion inhibitors for industries is often guided by the following principles
i. Based on the structure: compounds having hetero atoms in their aromatic system have been found to be good corrosion inhibitors for most metals [7-10].
ii. Some plant materials have been successfully utilized for the inhibition of the corrosion of most metals. Most inhibitors in this class contain phytochemicals such as tannin, saponin, glycoside, flavonoid, etc., hence their inhibition potentials are attributed to the presence of these phytochemicals [11-14].
We have also been found that drugs such as ampicillin, cloxacillin, sparfloxacin, norfloxacin, penicillin G, penicillin V potassium, ciprofloxacin, ampiclox, cloxacillin, chloramphenicol, amoxicillin, ofloxacin and tetracyline are good corrosion inhibitors for metals [15-23]. Our studies further revealed that inhibitors in this class contain hetero atoms or functional groups which enhanced their inhibition potentials. Our present study is aimed at investigating the inhibition potentials of lincomycin for the corrosion of zinc in H2SO4.
Lincomycin (2S,4R)-N-[(1R,2R)-2-hydroxy-1-[(2R,3R,4S,5R,6R)-3,4,5-trihydro-xy-6-(methylsulfanyl)oxan-2-yl]propyl]-1-methyl-4-propylpyrrolidine-2-carbo-xamide) belongs to the class of antibiotics called microlides. The chemical formula of lincomycin is C18H34N2O6S while its molar mass is 406.538 g/mol. The chemical structure of lincomycin is as shown below:
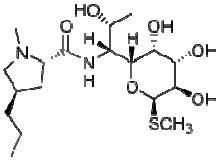
Chemical structure of lincomycin
From the above chemical structure, it can be seen that lincomycin has an aromatic system, hetero atoms (N, O) and functional groups that may facilitate its adsorption on zinc surface. Therefore, the objective of our study is to investigate the adsorption and inhibition potentials of lincomycin for the corrosion of zinc in various concentrations of H2SO4 and at various temperatures.
Experimental details
Materials
The material used for the study was zinc sheet which was mechanically pressed-cut into different coupons, each of dimension, 5x4x0.11 cm. Each coupon was degreased by washing with ethanol, dipped in acetone and allowed to dry in the air before it was preserved in a desiccator. All reagents used for the study were Analar grade and double distilled water was used for their preparation.
The inhibitor (lincomycin) was supplied by LIVEMOORE Pharmaceutical Company, Ikot Ekpene, Akwa Ibom State, Nigeria and was used without further purification. The concentrations of the inhibitors used for the study ranged from 0.0001 – 0.0005 M.
Gravimetric method
In the weight loss experiment, the pre-cleaned zinc coupon was dipped in 20 mL of the test solution maintained in a thermostated bath at 303, 313 and 323 K, respectively. The weight loss was determined by retrieving the coupon at 24 h interval progressively for 168 h (7 days). Prior to measurement, each coupon was washed in 5% chromic acid solution (containing 1% silver nitrate) and rinsed in deionized water. The difference in weight was taken as the weight loss of zinc.
From the weight loss measurements, the inhibition efficiency (%I) of the inhibitor, degree of surface coverage (q) and the corrosion rate (CR in g/h/cm2) of zinc were calculated using equations 1, 2 and 3, respectively.
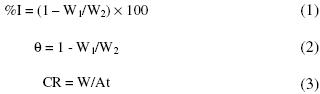
where W1 and W2 are the weight losses (g) for zinc in the presence and absence of the inhibitor in H2SO4 solution, q is the degree of surface coverage of the inhibitor, A is the area of the zinc coupon (in cm2), t is the period of immersion (in hours) and W is the weight loss of zinc steel after time, t.
Gasometric method
Gasometric methods were carried out at 303 K as described in the literature [24]. From the volume of hydrogen evolved per minute, inhibition efficiencies were calculated using equation 4.
![]()
where ![]() and
and ![]() are the volumes of H2 gas evolved at time ‘t’ for inhibited and uninhibited solutions, respectively.
are the volumes of H2 gas evolved at time ‘t’ for inhibited and uninhibited solutions, respectively.
Results and discussions
Effect of concentration of H2SO4 and lincomycin on the corrosion of Zn
Fig. 1 shows representative plots for the variation of weight loss with time during the corrosion of zinc in various concentrations of H2SO4 at 303 K. From the figure, it is evident that the weight loss of zinc increases with increase in the period of contact and with increasing concentration of H2SO4, indicating that the rate of corrosion of zinc in H2SO4 increases with increase in the concentration of H2SO4 and also with the period of contact. At various temperatures (plots not shown), weight losses of zinc were found to increase with increase in temperature, which also indicated that the rate of corrosion of zinc in solutions of H2SO4 also increases with increase in temperature.
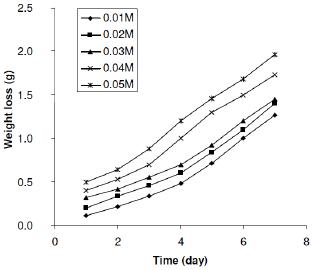
Figure 1. Variation of weight loss of Zn in various concentrations of tetraoxosulphate (VI) acid at 303 K.
In the presence of lincomycin, weight loss of zinc in H2SO4 decreases with increasing concentration of lincomycin, indicating that lincomycin is an adsorption inhibitor for the corrosion of zinc in H2SO4. Figs. 2a to 2c are representative plots (plots for other concentrations of H2SO4 not shown) for the variation of weight loss of Zn in 0.01 M H2SO4. From the figures, it can be seen that weight loss of zinc for the blank solutions is higher than those obtained for solutions of H2SO4 containing various concentrations of lincomycin. Therefore, lincomycin retarded the corrosion of zinc in H2SO4. Weight loss of zinc was also found to increase with increase in temperature implying that the inhibition efficiency of lincomycin for the corrosion of zinc decreases with increase in temperature.
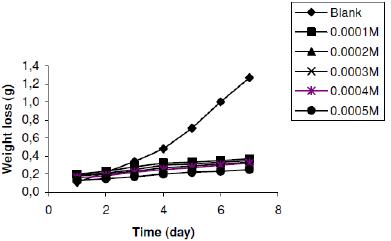
Figure 2a. Variation of weight loss with time for the corrosion of zinc in 0.01 M tetraoxosulphate (VI) acid containing various concentrations of lincomycin at 303 K.
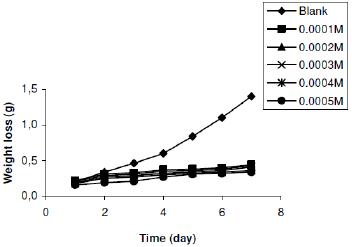
Figure 2b. Variation of weight loss with time for the corrosion of zinc in 0.02 M tetraoxosulphate (VI) acid containing various concentrations of lincomycin at 303 K.
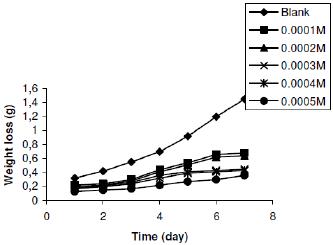
Figure 2c. Variation of weight loss with time for the corrosion of zinc in 0.03 M tetraoxosulphate (VI) acid containing various concentrations of lincomycin at 303 K.
Values of inhibition efficiency of lincomycin and the corrosion rates of zinc in various concentrations of H2SO4 are presented in Table 1. The results indicate that the corrosion rate of zinc increases with increase in temperature and with increasing concentration of H2SO4. However, the corrosion rate decreases with increasing concentration of lincomycin. These findings confirm that the rate of corrosion of zinc is dependent on temperature and concentration of the acid/inhibitor. Inhibition efficiencies obtained from gasometric analysis were 70.23, 84.34, 86.88, 90.02 and 94.34 % at lincomycin concentrations of 0.0001, 0.0002, 0.0003, 0.0004 and 0.0005 M respectively. These values are relatively higher than the average values obtained from weight loss measurements at 303 K, indicating that the instantaneous inhibition efficiency of lincomycin is better than its average inhibition efficiency.
Table 1. Corrosion rate (in gcm-2h-1) of zinc and inhibition efficiencies (%) of lincomycin in various concentrations of H2SO4.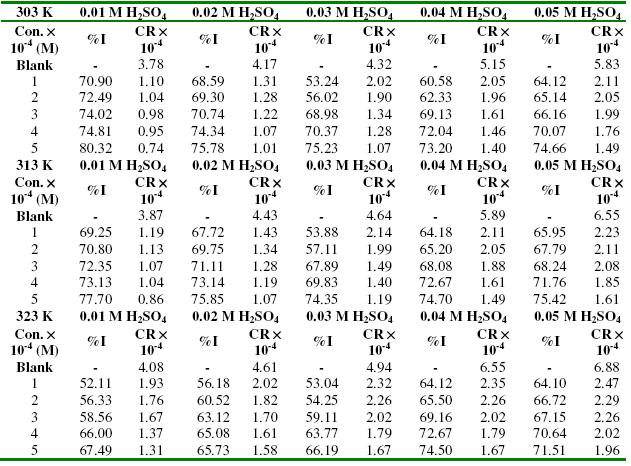
Effect of temperature
In order to study the effect of temperature on the corrosion of zinc, the Arrhenius equation was used (equation 5) [25],
![]()
where CR is the corrosion rates of zinc in gh-1cm-2, A is the Arrhenius constant, Ea is the activation energy in J/mol, R is the gas constant and T is the temperature in Kelvin. Using equation 5, the plots of logCR versus 1/T were linear for all concentrations of lincomycin and H2SO4 confirming the application of the Arrhenius equation to the corrosion of Zn in H2SO4. Figs. 3a to 3c are representative plots for the pattern of variation of logCR with 1/T.
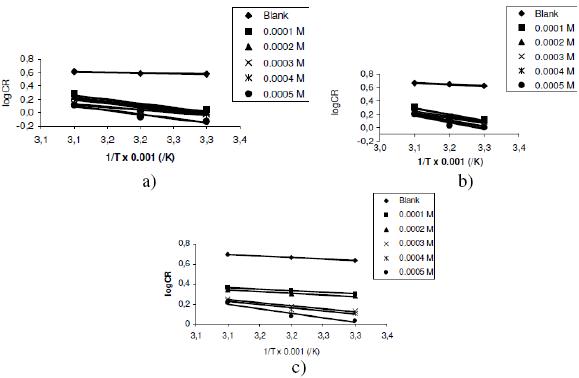
Figure 3. Variation of log(CR) with 1/T for the inhibition of the corrosion of Zn in (a) 0.01 M, (b) 0.02 M and (c) 0.03 M tetraoxosulphate(VI) acid by lincomycin.
Values of the Arrhenius constant and the activation energy deduced from the plots are presented in Table 2. From the results, it is evident that the degree of linearity of lines on the Arrhenius plots are very close to unity, indicating strong adherence of the experimental data to the assumptions of the Arrhenius theory. Secondly, values of the activation energies for the blank solutions were lower than those obtained for solutions containing lincomycin, indicating that lincomycin retarded the corrosion of zinc in H2SO4. Lastly, the activation energies were lower than the threshold value of 80 KJ/mol required for chemical adsorption, hence the adsorption of lincomycin on zinc surface is consistent with the mechanism of physical adsorption.
Table 2. Arrhenius constants and activation energies for the corrosion of zinc in 0.01 – 0.05 M H2SO4 in the presence of various concentrations of lincomycin.
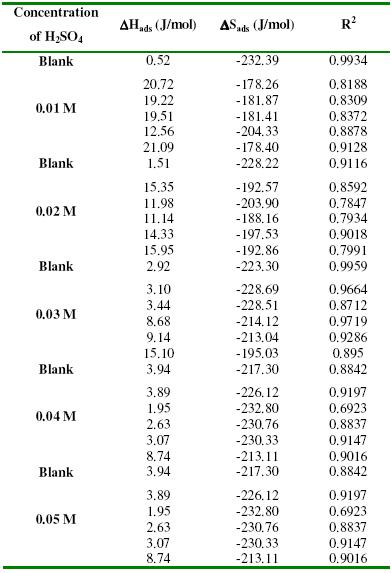
Thermodynamic/adsorption considerations
The transition state equation was used to calculate thermodynamic parameters for the adsorption of lincomycin on the surface of zinc. From the transition state equation, the relationship between the corrosion rate (CR) of zinc, enthalpy of adsorption ((DHads) and entropy of adsorption (DSads) can be written as follows [26-27]
![]()
where T is the temperature, N is the Avogadro’s number and h is the Planck constant. Rearranging and taking the logarithm of both sides of equation 6 yield equation 7:
![]()
From equation 7, a plot of log (CR/T) versus 1/T should produce a straight line with slope and intercept equal to DHads/2.303R and (log(R/Nh+DSads/2.303R), respectively. Figs. 4a to 4c illustrate the pattern of variation of the plots of log(CR/T) versus 1/T for 0.01 M H2SO4. At other concentrations of H2SO4, similar plots were obtained (not shown). However, in all cases, the degrees of linearity of the plots (R2) were very close to unity, indicating a strong adherence of the experimental data to the transition state theory. Values of DHads calculated from the slopes of the transition state plots are recorded in Table 3. These values are positive, indicating that the adsorption of lincomycin on zinc surface is endothermic. Also values of DSads are negative, implying that the adsorption of lincomycin on zinc surface is accompanied by increasing degree of orderliness.
Figure 4. Variation of log(CR/T) with 1/T for the inhibition of the corrosion of Zn in (a) 0.01 M, (b) 0.02 M and (c) 0.03 M tetraoxosulphate(VI) acid by lincomycin.
Table 3. Thermodynamic parameters for the adsorption of lincomycin on zinc surface immersed in 0.01 – 0.05 M H2SO4.
It is an established fact that corrosion inhibitors act by being adsorbed on the metal surface [28-29]. In order to study the adsorption characteristics of lincomycin on zinc surface, we fitted the data obtained for the degree of surface coverage of lincomycin (at different concentrations) into different adsorption isotherms. The tests indicated that Langmuir adsorption isotherm best describes the adsorption characteristics of lincomycin on zinc surface. The assumptions of Langmuir adsorption isotherm can be expressed as equation 8 [30-31]
![]()
where C is the concentration of the inhibitor on the bulk electrolyte, q is the degree of surface coverage of the inhibitor and K is the equilibrium constant of adsorption of the inhibitor.
The plots of log(C/q) versus logC were linear (Figs. 5a to 5c) implying that the experimental data is consistent with the theory of Langmuir adsorption isotherm. Values of adsorption parameters deduced from the Langmuir isotherms are presented in Table 4.
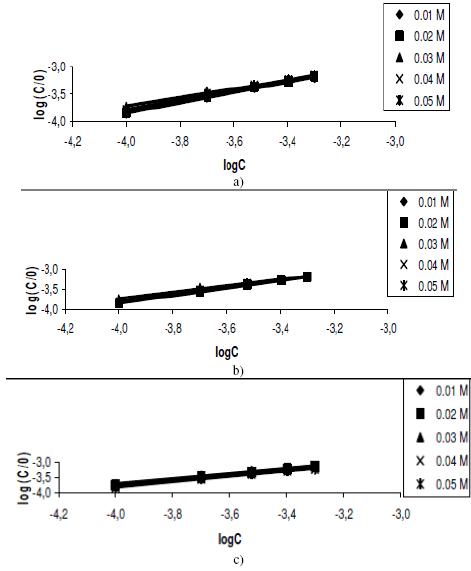
Figure 5. Langmuir isotherm for the adsorption of lincomycin on zinc surface in various concentrations of tetraoxosulphate (VI) acid at (a) 303 K, (b) 313 K and (c) 323 K.
Table 4. Langmuir adsorption parameters for the adsorption of lincomycin on zinc surface in various concentrations of H2SO4 and temperatures.
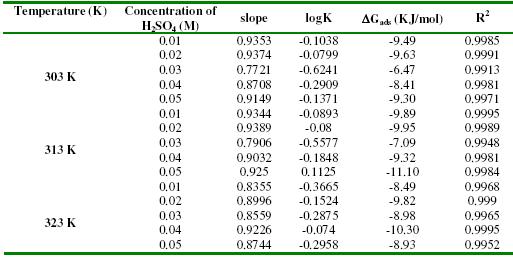
It is also significant to note that the slopes of the plots were very close to unity, implying that Langmuir adsorption isotherm strongly favoured the adsorption of lincomycin on zinc surface. Also, from the intercept of the Langmuir isotherms, values of K were calculated. These values fitted the model for the estimation of the free energies of adsorption of lincomycin on zinc surface as follows [32],
![]()
where DGads is the free energy of adsorption of the inhibitor, R is the gas constant and T is the temperature. From the results obtained, the activation energies are negatively less than the threshold value of -40 KJ/mol required for chemical adsorption. Therefore, the adsorption of lincomycin on zinc surface is spontaneous and is consistent with electrostatic interaction between charged molecules and charged metal surface and favours the mechanism of physical adsorption.
Conclusions
Lincomycin is a good adsorption inhibitor for the corrosion of zinc in 0.01 to 0.05 M H2SO4. The inhibitor acts by being adsorbed on zinc surface according to the mechanism of physical adsorption. The adsorption of the inhibitor is spontaneous and endothermic. Therefore, we recommend the use of lincomycin as an inhibitor for the corrosion of zinc in H2SO4.
References
1. S.K. Rajappa, T.V. Venkatesha, B.M. Praveen, Bull. Mater. Sci. 31 (2008) 37.
2. N.O. Eddy, A.S. Ekop, Mater. Sci. 4 (2008) 10.
3. S.A. Odoemelam, N.O. Eddy, Mater. Sci. 4 (2008) 1.
4. J. Fang, J. Lie, J. Mol. Struct. 593 (2002) 179.
5. Y.K. Agrawal, J.D. Talati, M.D. Shah, M.N. Desai, N.K. Shah, Corrosion Sci. 46 (2004) 633. [10.1016/S0010-938X(03)00174-4]
6. S. Rajendran, J. Jeyasundari, P. Usha, J.A. Selvi, B. Narayanasamy, A.P.P. Regis, P. Rengan, Port. Electrochim. Acta 27 (2009) 153. [10.4152/pea.200902153] [ Links ]
7. N.O. Eddy, U.J. Ibok, E.E. Ebenso, A. El Nemr, E.H. Ashry, J. Molec. Model. 15 (2009) 1085. [10.1007/s00894-009-0472-7]
8. S.A. Odoemelam, N.O. Eddy, J. Surf. Sci. Tech. 24 (2008) 65.
9. A. Yurt, G. Bereket, A. Kivrak, A. Balaban and B. Erk, J. Appl. Electrochem. 35 (2005) 1025. [10.1007/s10800-005-7336-3]
10. N.O. Eddy, S.A. Odoemelam, A.O. Odiongenyi, J. Appl. Electrochem. 39 (2009) 849. [10.1007/s10800-008-9731-z]
11. N.O. Eddy, S.A. Odoemelam, Pigment Resin Technol. 38 (2009) 111. [10.1108/03699420910940617]
12. E.E. Ebenso, N.O. Eddy, A.O. Odiongenyi, Afri. J. Pure Appl. Chem. 4 (2008) 107.
13. A.O. Odiongeyi, S.A. Odoemelam, N.O. Eddy, Port. Electrochim. Acta 27 (2009) 33. [10.4152/pea.200901033] [ Links ]
14. N.O. Eddy and E.E. Ebenso, African J. Pure Appl. Chem. 2 (2008) 1.
15. N.O. Eddy, S.A. Odoemelam, P.A. Ekwumemgbo, Sci. Res. Essay 4 (2009) 33.
16. E.E. Ebenso, N.O. Eddy, A.O. Odiongenyi, Port. Electrochim. Acta 27 (2009) 13. [10.4152/pea.200901013] [ Links ]
17. S.A. Odoemelam, E.C. Ogoko, B.I. Ita, N.O. Eddy, Port. Electrochim. Acta 27 (2009) 57. [10.4152/pea.200901057] [ Links ]
18. N.O. Eddy, S.A. Odoemelam, A.J. Mbaba, J. Pure Appl. Chem. 2 (2008) 132.
19. N.O. Eddy, S.A. Odoemelam, Internat. J. Pure Appl. Chem. 3 (2009) 1.
20. N.O. Eddy, S.A. Odoemelam, N.W. Akpanudoh, Research J. Pure Appl. Sci. 4 (2009) 1963.
21. N.O. Eddy and E.E. Ebenso, J. Molecular Modeling (2010). [10.1007/S00894-0090635-6]
22. N.O. Eddy, S.A. Odoemelam, N.W. Akpanudoh, J. Chem. Technol. 2 (2008) 1.
23. N.O. Eddy, U.J. Ibok and E.E. Ebenso, J. Applied. Electrochemistry 40 (2010) 445-456.
24. E.E. Oguzie, B.N. Okolue, E.E. Ebenso, G.N. Onuoha, A.I. Onochukwu, Mater. Chem. Phys. 87 (2004) 394. [10.1016/j.matchemphys.2004.06.003]
25. M. Abdallah, Corrosion Sci. 44 (2002) 717. [10.1016/S0010-938X(01)00100-7]
26. M. Abdallah, Port. Electrochim. Acta 22 (2004) 161. [10.4152/pea.200402161] [ Links ]
27. N.O. Eddy, “Inhibition of corrosion of mild steel by some antibiotics”, Ph.D. Thesis, University of Calabar, Nigeria, 2008.
28. G.K. Gomma, Mater. Chem. Phys. 55 (1998) 241. [10.1016/S0254-0584(98)00155-2]
29. S. Acharya, S. N. Upadhyay, Trans. Indian Inst. Met. 57 (2004) 297.
30. S.T. Arab, A.M. Al-Turkustani, Port. Electrochim. Acta 24 (2006) 53. [10.4152/pea.200601053] [ Links ]
31. M.M. Attar, J.D. Scantlebury, JCSE 8(1) (2005) 97.
32. M.A. Bendahou, M.B.E. Benadellah, B.B. Hammouti, Pigment Resin Technol. 35 (2006) 95. [10.1108/03699420610652386]
Received 18 April 2009; accepted 27 April 2010
*Corresponding author: nabukeddy@yahoo.com














